Table 1.
Formal [2 + 2 + 2] Cycloadditions via Propargylic Ene/Cyano Diels-Alder Reactions
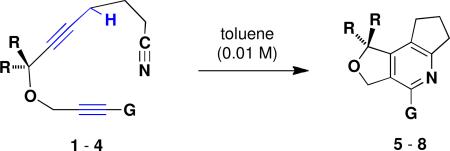 | ||||||
|---|---|---|---|---|---|---|
| entry | R | G | conditions | product | yield (%)a | |
| 1 | 1 | H | H | 160 °C, 21 h | 5 | 71 |
| 2 | 2 | CH3 | H | reflux, 66 h | 6 | 96 |
| 3 | 2 | CH3 | H | 140 °C, 18 h | 6 | 55 |
| 4 | 3 | H | C≡CSiMe3 | reflux, 24 h | 7 | 30 |
| 5 | 4 | H | C≡CCH2OSit-BuMe2 | reflux, 46 h | 8 | 37 |
Isolated yield of products purified by chromatography.
