Abstract
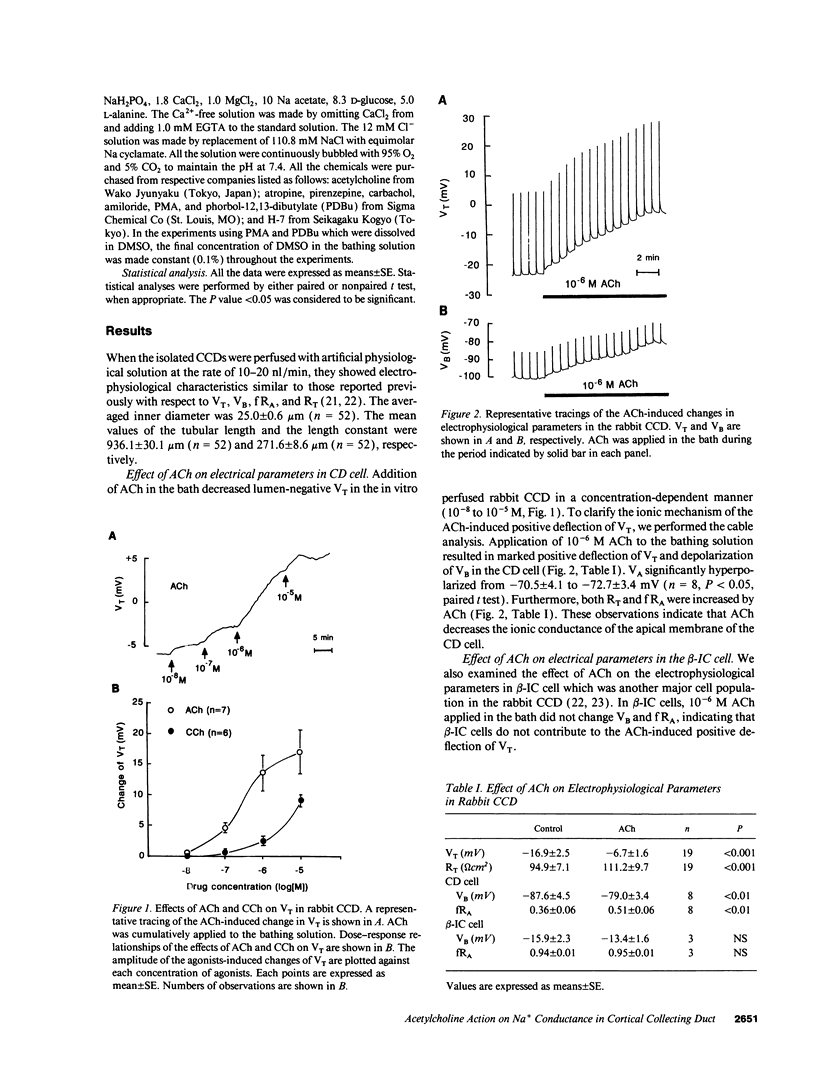
We examined effects of acetylcholine (ACh) on the electrical parameters and intracellular Ca2+ concentration ([Ca2+]i) in the isolated rabbit cortical collecting duct (CCD) perfused in vitro using the conventional microelectrode technique and microscopic fluorescence spectrophotometry. ACh (10(-8) to 10(-5) M) in the bath caused a positive deflection of the transepithelial voltage (VT) and an increase in [Ca2+]i. Carbachol also showed similar but smaller effects. The effects of ACh were antagonized by muscarinic receptor antagonists. ACh at 10(-6) M hyperpolarized the apical membrane voltage and increased the fractional resistance of the apical membrane of the collecting duct cells accompanied by a positive deflection of VT and an increase in transepithelial resistance, whereas it did not affect these parameters in the beta-intercalated cells. In the presence of 10(-5) M amiloride in the lumen, the effects of ACh were almost completely abolished. The ACh-induced increase in [Ca2+]i is accounted for by the release of Ca2+ from intracellular store and Ca2+ entry from the bath. In the absence of Ca2+ in the bath, the ACh-induced changes in electrophysiological parameters were significantly smaller than those observed in the presence of Ca2+. Both phorbol-12-myristate-13-acetate (PMA) and phorbol-12,13-dibutylate (PDBu), activators of protein kinase C (PKC), also inhibited the apical Na+ conductance. In the presence of PMA or PDBu in the bath, ACh did not show further inhibitory effect. 1-(5-Isoquinolinylsulfonyl)-2-methylpiperazine, an inhibitor of PKC, partially attenuated the effect of ACh. These observations indicate that ACh inhibits the apical Na+ conductance partly by both increasing [Ca2+]i and activating PKC. Such an action of ACh may partially explain its natriuretic effect.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyenbach K. W., Frömter E. Electrophysiological evidence for Cl secretion in shark renal proximal tubules. Am J Physiol. 1985 Feb;248(2 Pt 2):F282–F295. doi: 10.1152/ajprenal.1985.248.2.F282. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Cohen M. E., Wesolek J., McCullen J., Rys-Sikora K., Pandol S., Rood R. P., Sharp G. W., Donowitz M. Carbachol- and elevated Ca(2+)-induced translocation of functionally active protein kinase C to the brush border of rabbit ileal Na+ absorbing cells. J Clin Invest. 1991 Sep;88(3):855–863. doi: 10.1172/JCI115387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Michele M., Amenta F., Cavallotti C. Autoradiographic localization of muscarinic receptors within the rat kidney. Eur J Pharmacol. 1989 Oct 10;169(2-3):297–305. doi: 10.1016/0014-2999(89)90027-7. [DOI] [PubMed] [Google Scholar]
- Frindt G., Windhager E. E. Ca2(+)-dependent inhibition of sodium transport in rabbit cortical collecting tubules. Am J Physiol. 1990 Mar;258(3 Pt 2):F568–F582. doi: 10.1152/ajprenal.1990.258.3.F568. [DOI] [PubMed] [Google Scholar]
- Goyal R. K. Muscarinic receptor subtypes. Physiology and clinical implications. N Engl J Med. 1989 Oct 12;321(15):1022–1029. doi: 10.1056/NEJM198910123211506. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hayashi M., Yamaji Y., Iyori M., Kitajima W., Saruta T. Effect of isoproterenol on intracellular pH of the intercalated cells in the rabbit cortical collecting ducts. J Clin Invest. 1991 Apr;87(4):1153–1157. doi: 10.1172/JCI115112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays S. R., Baum M., Kokko J. P. Effects of protein kinase C activation on sodium, potassium, chloride, and total CO2 transport in the rabbit cortical collecting tubule. J Clin Invest. 1987 Dec;80(6):1561–1570. doi: 10.1172/JCI113242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hébert R. L., Jacobson H. R., Breyer M. D. Prostaglandin E2 inhibits sodium transport in rabbit cortical collecting duct by increasing intracellular calcium. J Clin Invest. 1991 Jun;87(6):1992–1998. doi: 10.1172/JCI115227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino Y., Imai M. Effects of prostaglandins on Na transport in isolated collecting tubules. Pflugers Arch. 1978 Feb 22;373(2):125–132. doi: 10.1007/BF00584850. [DOI] [PubMed] [Google Scholar]
- Koeppen B. M., Biagi B. A., Giebisch G. H. Intracellular microelectrode characterization of the rabbit cortical collecting duct. Am J Physiol. 1983 Jan;244(1):F35–F47. doi: 10.1152/ajprenal.1983.244.1.F35. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Yoshitomi K., Ikeda M., Uchida S., Naruse M., Imai M. Regulation of cortical collecting duct function: effect of endothelin. Am Heart J. 1993 Feb;125(2 Pt 2):582–588. doi: 10.1016/0002-8703(93)90207-p. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Peralta E., Clapham D. Diverse functions of muscarinic acetylcholine receptor subtypes. Trends Pharmacol Sci. 1989 Dec;Suppl:34–38. [PubMed] [Google Scholar]
- Ling B. N., Eaton D. C. Effects of luminal Na+ on single Na+ channels in A6 cells, a regulatory role for protein kinase C. Am J Physiol. 1989 Jun;256(6 Pt 2):F1094–F1103. doi: 10.1152/ajprenal.1989.256.6.F1094. [DOI] [PubMed] [Google Scholar]
- Ling B. N., Kokko K. E., Eaton D. C. Inhibition of apical Na+ channels in rabbit cortical collecting tubules by basolateral prostaglandin E2 is modulated by protein kinase C. J Clin Invest. 1992 Oct;90(4):1328–1334. doi: 10.1172/JCI115998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti J., Taniguchi S., Lebrun F., Morel F. Cholinergic agonists increase cell calcium in rat medullary collecting tubules. A fura-2 study. Pflugers Arch. 1990 Jul;416(5):561–567. doi: 10.1007/BF00382690. [DOI] [PubMed] [Google Scholar]
- McArdle S., Garg L. C., Crews F. T. Cholinergic receptors in renal medullary collecting duct cells. J Pharmacol Exp Ther. 1989 Jan;248(1):12–16. [PubMed] [Google Scholar]
- McArdle S., Garg L. C., Crews F. T. Cholinergic stimulation of phosphoinositide hydrolysis in rabbit kidney. J Pharmacol Exp Ther. 1988 Feb;244(2):586–591. [PubMed] [Google Scholar]
- Muto S., Furuya H., Tabei K., Asano Y. Site and mechanism of action of epidermal growth factor in rabbit cortical collecting duct. Am J Physiol. 1991 Feb;260(2 Pt 2):F163–F169. doi: 10.1152/ajprenal.1991.260.2.F163. [DOI] [PubMed] [Google Scholar]
- Muto S., Yasoshima K., Yoshitomi K., Imai M., Asano Y. Electrophysiological identification of alpha- and beta-intercalated cells and their distribution along the rabbit distal nephron segments. J Clin Invest. 1990 Dec;86(6):1829–1839. doi: 10.1172/JCI114913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pirola C. J., Alvarez A. L., Balda M. S., Finkielman S., Nahmod V. E. Evidence for cholinergic innervation in dog renal tissue. Am J Physiol. 1989 Nov;257(5 Pt 2):F746–F754. doi: 10.1152/ajprenal.1989.257.5.F746. [DOI] [PubMed] [Google Scholar]
- Pirola C. J., Alvarez A. L., Finkielman S., Nahmod V. E. Release of acetylcholine from isolated canine renal tissue. Am J Physiol. 1991 Feb;260(2 Pt 2):F198–F203. doi: 10.1152/ajprenal.1991.260.2.F198. [DOI] [PubMed] [Google Scholar]
- Sackin H., Boulpaep E. L. Isolated perfused salamander proximal tubule: methods, electrophysiology, and transport. Am J Physiol. 1981 Jul;241(1):F39–F52. doi: 10.1152/ajprenal.1981.241.1.F39. [DOI] [PubMed] [Google Scholar]
- Sansom S. C., Weinman E. J., O'Neil R. G. Microelectrode assessment of chloride-conductive properties of cortical collecting duct. Am J Physiol. 1984 Aug;247(2 Pt 2):F291–F302. doi: 10.1152/ajprenal.1984.247.2.F291. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J., Barasch J., Al-Awqati Q. Plasticity of functional epithelial polarity. 1985 Nov 28-Dec 4Nature. 318(6044):368–371. doi: 10.1038/318368a0. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Naruse M., Takeda M., Nakamura M., Yoshitomi K., Imai M. Mechanism of PGE2-induced cell swelling in distal nephron segments. Am J Physiol. 1992 Nov;263(5 Pt 2):F824–F832. doi: 10.1152/ajprenal.1992.263.5.F824. [DOI] [PubMed] [Google Scholar]
- Snyder H. M., Fredin D. M., Breyer M. D. Muscarinic receptor activation inhibits AVP-induced water flow in rabbit cortical collecting ducts. Am J Physiol. 1991 Jun;260(6 Pt 2):F929–F936. doi: 10.1152/ajprenal.1991.260.6.F929. [DOI] [PubMed] [Google Scholar]
- Takeda M., Yoshitomi K., Imai M. Regulation of Na(+)-3HCO3- cotransport in rabbit proximal convoluted tubule via adenosine A1 receptor. Am J Physiol. 1993 Oct;265(4 Pt 2):F511–F519. doi: 10.1152/ajprenal.1993.265.4.F511. [DOI] [PubMed] [Google Scholar]
- Taniguchi S., Marchetti J., Morel F. Cytosolic free calcium in single microdissected rat cortical collecting tubules. Pflugers Arch. 1989 Jun;414(2):125–133. doi: 10.1007/BF00580953. [DOI] [PubMed] [Google Scholar]
- VANDER A. J. EFFECTS OF ACETYLCHOLINE, ATROPINE, AND PHYSOSTIGMINE ON RENAL FUNCTION IN THE DOG. Am J Physiol. 1964 Mar;206:492–498. doi: 10.1152/ajplegacy.1964.206.3.492. [DOI] [PubMed] [Google Scholar]
- Vehaskari V. M., Herndon J., Hamm L. L. Mechanism of sodium transport inhibition by epidermal growth factor in cortical collecting ducts. Am J Physiol. 1991 Nov;261(5 Pt 2):F896–F903. doi: 10.1152/ajprenal.1991.261.5.F896. [DOI] [PubMed] [Google Scholar]
- Warden D. H., Stokes J. B. EGF and PGE2 inhibit rabbit CCD Na+ transport by different mechanisms: PGE2 inhibits Na(+)-K+ pump. Am J Physiol. 1993 Apr;264(4 Pt 2):F670–F677. doi: 10.1152/ajprenal.1993.264.4.F670. [DOI] [PubMed] [Google Scholar]
- Wiesmann W., Sinha S., Yates J., Klahr S. Cholinergic agents inhibit sodium transport across the isolated toad bladder. Am J Physiol. 1978 Dec;235(6):F564–F569. doi: 10.1152/ajprenal.1978.235.6.F564. [DOI] [PubMed] [Google Scholar]
- Yanase M., Handler J. S. Activators of protein kinase C inhibit sodium transport in A6 epithelia. Am J Physiol. 1986 Mar;250(3 Pt 1):C517–C522. doi: 10.1152/ajpcell.1986.250.3.C517. [DOI] [PubMed] [Google Scholar]
- Yoshitomi K., Koseki C., Taniguchi J., Imai M. Functional heterogeneity in the hamster medullary thick ascending limb of Henle's loop. Pflugers Arch. 1987 May;408(6):600–608. doi: 10.1007/BF00581162. [DOI] [PubMed] [Google Scholar]
- Yun J. C., Oriji G., Gill J. R., Jr, Coleman B. R., Peters J., Keiser H. Role of muscarinic receptors in renal response to acetylcholine. Am J Physiol. 1993 Jul;265(1 Pt 2):F46–F52. doi: 10.1152/ajprenal.1993.265.1.F46. [DOI] [PubMed] [Google Scholar]


