Scheme 1.
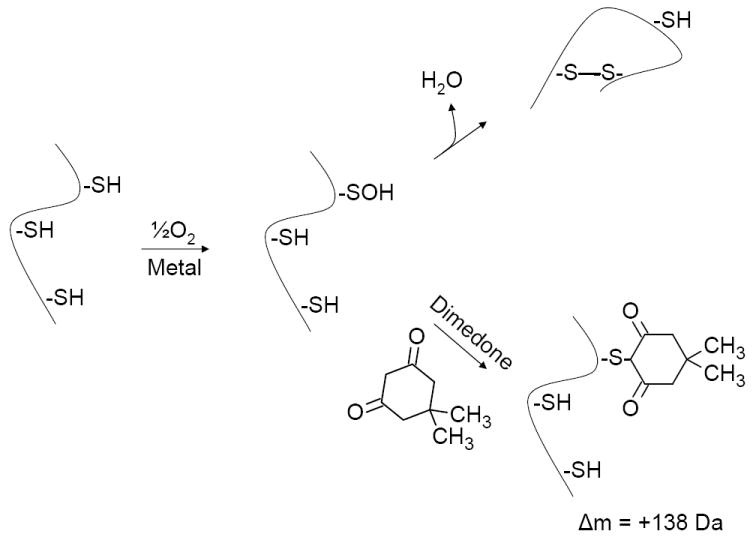
Proposed reaction pathway for in vitro generation of disulfide bonds and protein folding. Dimedone reacts covalently and irreversibly with Cys-SOH, making it an effective probe for the existence of Cys-SOH (10, 32-39). Detection of covalent dimedone-modified protein in the experiments described here indicates the production of protein Cys-SOH. By logical extension of previous studies on the reactivity of intra- and intermolecular sulfhydryl groups with Cys-SOH (7, 40), such detection suggests that Cys-SOH is an oxidized sulfur intermediate that mediates in vitro disulfide bond formation and protein folding. Early experiments by Anfinsen and colleagues (2-4, 6) showed that in vitro protein folding goes to completion and full restoration of protein activity.
