FIGURE 6.
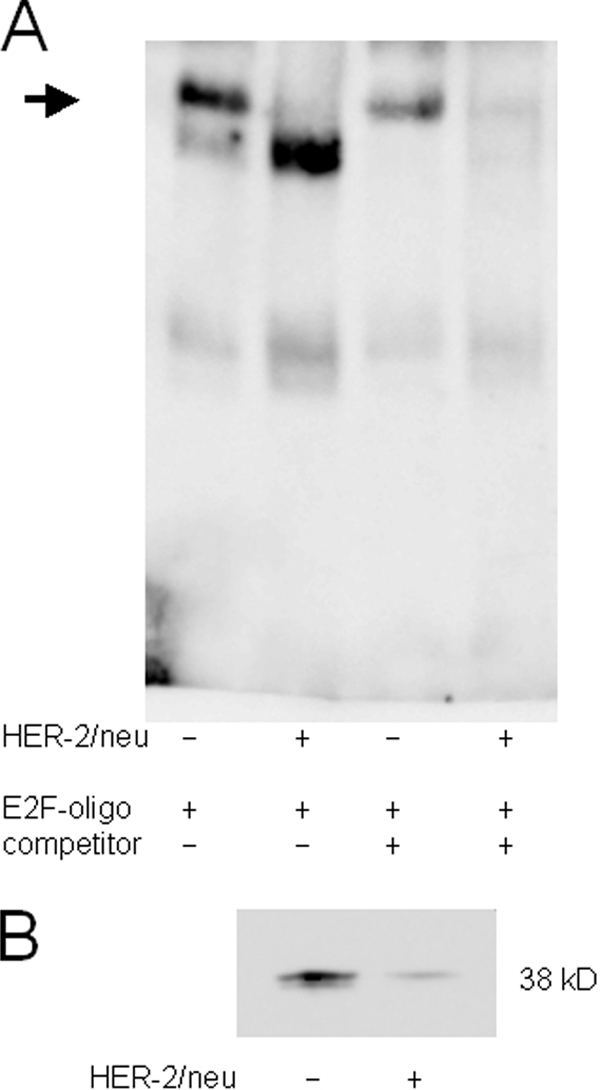
Different binding properties for distal E2F motif in the tapasin promoter. A, electrophoretic mobility shift assay for the analysis of the proximal E2F(−146)-binding site. EMSA was performed as described under “Experimental Procedures” using a biotin-labeled oligonucleotide spanning the tapasin promoter sequence from −146 to −139 (supplemental Table 1). All lanes contain 100 pg of labeled oligonucleotide. The arrow indicates different E2F binding properties in crude nuclear extracts of HER-2/neu− and HER-2/neu+ cells. The specific signal in HER-2/neu+ cells disappeared by adding a 100-fold excess of the respective unlabeled oligonucleotide, whereas the signal intensity is reduced in HER-2/neu− cells. B, a distinct 38-kDa protein bound to the labeled E2F probe in Southwestern blot of nuclear extracts from HER-2/neu− and HER-2/neu+ cells. The Southwestern blot was probed with the biotinylated oligonucleotide containing the proximal E2F(−146)-binding site from the tapasin promoter. An increased amount of labeled oligonucleotide bound to proteins from nuclear extracts of HER-2/neu− cells when compared with HER-2/neu+ cells.
