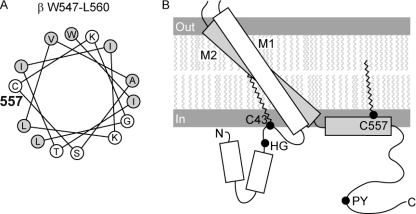
FIGURE 7.
Predicted locations of palmitoylated βC43 and βC557. The presence of α-helices in the cytoplasmic domains was predicted using the PROF program of cascaded multiple classifiers to predict secondary structure (available on-line). A, palmitoylated βC557 is within an α-helix at the junction of the C-terminal cytoplasmic domain and M2. Placement of residues in the C-terminal cytoplasmic α-helix is shown as a helical wheel. Hydrophobic residues are indicated by shaded circles; hydrophilic residues are indicated by open circles. See text for discussion. B, rectangles represent α-helices. The rotation, angle, and length of the rectangles for M1 and M2 are based on the crystal structure of ASIC1. Palmitoylated βC43 in the N-terminal cytoplasmic domain is found in a coil domain between M1 and a cytoplasmic α-helix. The His-Gly (HG) and Pro-Tyr (PY) motifs are located at the indicated positions. Palmitate is indicated within the inner leaflet of the membrane as wavy lines.