Abstract
Bacterial acyl carrier protein (ACP) is a highly anionic, 9 kDa protein that functions as a cofactor protein in fatty acid biosynthesis. Escherichia coli ACP is folded at neutral pH and in the absence of divalent cations, while Vibrio harveyi ACP, which is very similar at 86% sequence identity, is unfolded under the same conditions. V. harveyi ACP adopts a folded conformation upon the addition of divalent cations such as Ca2+ and Mg2+ and a mutant, A75H, was previously identified that restores the folded conformation at pH 7 in the absence of divalent cations. In this study we sought to understand the unique folding behavior of V. harveyi ACP using NMR spectroscopy and biophysical methods. The NMR solution structure of V. harveyi ACP A75H displays the canonical ACP structure with four helices surrounding a hydrophobic core, with a narrow pocket closed off from the solvent to house the acyl chain. His-75, which is charged at neutral pH, participates in a stacking interaction with Tyr-71 in the far C-terminal end of helix IV. pH titrations and the electrostatic profile of ACP suggest that V. harveyi ACP is destabilized by anionic charge repulsion around helix II that can be partially neutralized by His-75 and is further reduced by divalent cation binding. This is supported by differential scanning calorimetry data which indicate that calcium binding further increases the melting temperature of V. harveyi ACP A75H by ∼20 °C. Divalent cation binding does not alter ACP dynamics on the ps-ns timescale as determined by 15N NMR relaxation experiments, however, it clearly stabilizes the protein fold as observed by hydrogen-deuterium exchange studies. Finally, we demonstrate that the E. coli ACP H75A mutant is similarly unfolded as wild-type V. harveyi ACP, further stressing the importance of this particular residue for proper protein folding.
Keywords: Calcium, Fatty Acid Synthase, NMR, Protein Folding, Protein Stability, Acyl Carrier Protein, Magnesium, Protein Dynamics
Introduction
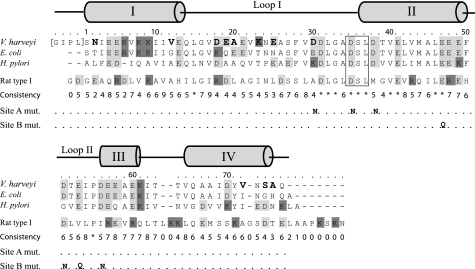
Bacterial acyl carrier proteins (ACP)2 are small, acidic proteins that are best known for their function as cofactor proteins in fatty acid (FA) biosynthesis. They contrast mammalian, type I ACPs in that they are discrete proteins, rather than part of a large, multi-enzyme polypeptide chain (1, 2). ACP functions as a shuttle that covalently binds all FA intermediates and delivers them to the enzymes of the FA synthase (FAS) system and ultimately delivers the majority of the FAs for membrane biosynthesis (3, 4). The FAs are bound via a phosphopantetheine prosthetic group that is attached to a conserved serine residue by ACP synthase (ACPS), converting apo- into holo-ACP. The prosthetic group is derived from coenzyme A and ends in a sulfhydryl that forms a thioester bond with the growing acyl chain during the elongation process. FA elongation takes place in a cyclic fashion elongating the acyl chain in two carbon unit intervals (2, 5). The highly dynamic nature of ACP is thought to be important for its ability to reversibly interact with the large variety of proteins involved in FA synthesis and for the propensity of ACP to readily hide and present bound acyl chain substrates (3). The structure of ACP is well conserved and consists of a four helix bundle, of which α-helices I, II, and IV run almost parallel to each other, while α-helix III is shorter and lies almost perpendicular to the other helices (Fig. 1) (2, 3). A hydrophobic pocket lies in the center of the three major helices where the growing, hydrophobic acyl chain is protected from the solvent when ACP is not interacting with FAS enzymes (6–8). During its interactions with enzymes of the FAS system, ACP must extrude the acyl chain from within its hydrophobic pocket and direct it into the active site of the attached protein (2, 5). In this process, helix II of ACP is thought to be critical for ACP recognition and consequently has been termed the recognition helix (9). Although no consensus interaction motif has been found on the enzymes that bind ACP, a basic/hydrophobic patch next to the active site has been identified on several of these proteins and is believed to be important for the correct association with the carrier protein (10). Helix II is also the site of two divalent cation-binding sites on ACP. In E. coli ACP (EcACP), site A is thought to consist of Glu-30, Asp-35, and Asp-38, which is located at the N-terminal end of helix II, while site B is composed of Glu-47, Asp-51, Glu-53, and Asp-56 at the opposite end of the helix and leading into loop II (11).
FIGURE 1.
Protein sequence alignment of VhACP and other related ACPs. The numbering is performed according to the V. harveyi sequence and begins at Ser-1, which is preceded by a four residue extension present because of the cloning protocol. Anionic residues are highlighted in light gray, and cationic residues are shown in dark gray. The phosphopantetheine attachment site is boxed, and a secondary structure diagram is provided above the sequences. The differences in primary structure between the E. coli and V. harveyi ACP sequences are highlighted in bold. The residues mutated for the calcium-binding site A and B elimination mutants are also shown.
The marine organism V. harveyi possesses a dissociated, type II ACP of 76 amino acids and a low pI of 4.1 that not only provides FAs for membrane synthesis, but also for bioluminescence (12, 13). Despite a high amino acid sequence identity (86%, Fig. 1), V. harveyi ACP (VhACP) is unfolded while EcACP is fully folded at pH 7 (14, 15). Intermediate to these two extremes, Helicobacter pylori ACP is partially folded under the same circumstances (16). As such, VhACP has been described as a natively unfolded protein, in particular because it has recently been shown that folding can be induced by binding to FAS enzymes (3, 17). Various other factors have also been found to stabilize VhACP and result in protein folding. Binding of the divalent cations magnesium or calcium, as well as attachment of acyl chain substrates, restores the folded form of VhACP and stabilizes it against alkaline denaturation (12, 14, 18). In addition to these external factors, the mutation of a single VhACP residue, Ala-75, to its corresponding residue in EcACP, His-75, is capable of inducing the folded conformation (15). Furthermore, mutants that neutralize the divalent cation-binding sites A and B of VhACP (Fig. 1) have been made that together (i.e. both binding sites abolished) remove the requirement for divalent cations for folding (18).
To investigate the role of His-75 in the VhACP A75H mutant and to determine how it is capable of altering the VhACP properties, we performed solution NMR studies to determine the mutant protein three-dimensional structure. The effect of divalent cation binding was investigated by NMR titration experiments, differential scanning calorimetry, as well as 15N NMR relaxation and hydrogen-deuterium exchange experiments. The VhACP divalent cation-binding site mutants A and B were also used to investigate calcium binding. Furthermore, a number of additional E. coli and V. harveyi ACP single mutants were constructed and biophysically characterized to aid in the understanding of the stabilizing and destabilizing forces involved in the proper folding of ACP.
EXPERIMENTAL PROCEDURES
Cloning and Site-directed Mutagenesis of V. harveyi and E. coli ACPs
Wild-type VhACP and VhACP A75H plasmids in the pGEX-5X-3 vector were obtained as previously described in Refs. 14, 15. The use of the pGEX system allows for the expression and purification of a glutathione S-transferase (GST)-ACP fusion protein. The wild-type vector was used to generate two VhACP substitution mutants, Y71W and A75W. All PCR-based site-directed mutagenesis, including mutagenic primer design, was performed using the protocol outlined in the QuikChange site-directed mutagenesis system (Stratagene). Mutant sequences were confirmed by DNA sequencing.
A synthetic E. coli ACP (EcACP) gene as described in Ref. 19 was cloned into the pGEX-5X-3 vector (Amersham Biosciences) using 5′ BamHI and 3′ XhoI restriction sites. This vector was subsequently used as a template for site-directed mutagenesis to construct the EcACP H75A mutant. The cloning vectors were maintained in E. coli DH5α cells, and mutations were confirmed using DNA sequencing. For protein expression, the plasmids were transformed into E. coli BL21 cells.
ACP Protein Purification
E. coli and V. harveyi ACPs were produced using methods previously described (14, 18). Briefly, the E. coli cells were grown in 1L of LB growth media containing 100 mg/liter ampicillin, and protein expression was induced with 0.2 mm IPTG at an A600 ∼0.8. To produce 15N-labeled VhACP A75H, bacteria were grown in M9 minimal media containing 0.5 g/liter 15NH4Cl. The GST-ACP cell extract was incubated with crudely purified E. coli ACPS for complete conversion of apo- to holo-ACP (20). The GST-ACP protein was purified using a glutathione-Sepharose 4B column, followed by Factor Xa cleavage of the GST tag. This yields ACP containing a four residue, N-terminal extension of GIPL. The proteins were further purified on an Äkta Purifier (Pharmacia) using a RESOURCEQ anion exchange column (Amersham Biosciences), in 10 mm MES pH 6.0. Elution was achieved using a combination of linear and stepwise gradients up to 0.45 m NaCl in the above buffer. The presence of holo-ACP was confirmed either using native gel electrophoresis (21) or matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) through the SAMS center at the University of Calgary. The VhACP metal binding site mutant proteins A and B were prepared as described in (18). The concentration of ACP was determined via A280 absorbance using extinction coefficients obtained from the ExPASy ProtParam program.
NMR Spectroscopy
V. harveyi holo-ACP A75H was dissolved after lyophilization or dialyzed against 10 mm KH2PO4 and concentrated to a final volume of 0.5 ml using MicroSEP 1 kDa cut-off spin columns. The final sample used for NMR commonly contained ∼1 mm protein, 10 mm deuterated DTT, 10% D2O, and 1% sodium azide. DSS was added to 0.1–0.5 mm as an internal standard, and the sample pH was adjusted to 5.7. Two-dimensional NOESY, TOCSY, and DQF-COSY proton spectra were collected at 25 °C on a Bruker Avance 700 MHz NMR spectrometer equipped with a 5 mm TBI triple axis-gradient probe. The number of data points were 2048 × 600, 2048 × 512, and 2048 × 527 in the directly and indirectly detected dimensions for the NOESY, TOCSY, and COSY spectra, respectively. The mixing times were 200 and 120 ms for the NOESY and TOCSY spectra, respectively, while all spectra had a sweep width of 8389.3 Hz in both dimensions. Water suppression was achieved using excitation sculpting (22). The three-dimensional, 15N-edited NOESY spectrum was acquired with a 200 ms mixing time and 1024 × 120 × 36 data points in the directly, indirectly detected and 15N detected dimensions. The sweep widths for the three-dimensional spectrum were 9766 Hz in the proton dimensions and 1395 Hz in the 15N dimension. All NMR data were processed using NMRPipe 3.4 and NMRDraw (23).
pH and calcium titrations of uniformly 15N-labeled VhACP A75H were conducted on a 700 MHz Bruker Avance NMR spectrometer (see above). For the divalent metal ion titrations, the protein was dialyzed into 20 mm Hepes buffer and residual calcium was removed using a BAPTA calcium sponge column (Invitrogen). Subsequently, 10 mm d-DTT, 0.1 mm DSS, and 0.03% sodium azide were added, and the protein was concentrated and the pH titrated to 7.0 using diluted HCl and NaOH. Each HSQC acquired contained 1024 × 120 data points in the proton and 15N dimensions, and the sweepwidths were 14 and 30 ppm, respectively.
For hydrogen/deuterium (H/D) exchange experiments, lyophilized VhACP A75H was dissolved in 20 mm Hepes in 100% D2O, also containing 10 mm d-DTT, 0.1 mm DSS, and 0.03% sodium azide. The pD was 6.6 based on pD = pH + 0.4 of the deuterium sample. The sample also contained either 5 mm CaCl2 or 0.1 mm EDTA (18). After dissolving the protein, data acquisition was performed approximately after 10 min, allowing for preparation of the spectrometer and sample-dependent experimental parameters. HSQC spectra were acquired using 8 scans and 2048 × 100 data points and sweepwidths of 12 and 25 ppm in the proton and 15N dimensions, respectively.
Natural abundance 15N-HSQCs of wild-type VhACP at pH 7.35 in the presence of 10 equivalents of either MgCl2 or CaCl2 were acquired on the 500 MHz spectrometer equipped with a cryoprobe as described above. The HSQCs contained 2048 × 128 data points and sweepwidths of 11 and 25 ppm in the proton and 15N dimensions, respectively. The spectra were collected using 512 scans. Average chemical shift differences of the peaks in the HSQC spectra were calculated according to Δδave = [(ΔδH2 + ΔδN2/25)/2]0.5.
The VhACP site A and B mutants were dialyzed into H2O, and residual calcium was removed using a BAPTA calcium sponge column (Invitrogen). As above, 10% D2O, 10 mm deuterated DTT, 0.5 mm DSS, and 0.03% sodium azide were added, and the pH adjusted to 5.7. At each point of the titration one-dimensional proton NMR spectra were acquired using excitation sculpting to suppress the water signal (22). Each spectrum consists of 256 scans containing 16384 data points and a sweep width of 8012 Hz on a Bruker Avance 500 MHz spectrometer equipped with a 5 mm TXI cryoprobe with a z-gradient.
NMR Structure Determination
The processed spectra were visualized using NMRView 5.0.4 (24). Proton chemical shift assignments were performed according to well established methods and general chemical shift ranges were taken from Wüthrich (25). The three-dimensional NOESY was used to resolve ambiguities in regions of spectral overlap in the 2D NOESY, however, the two-dimensional data were used for structure calculation. Structure calculations were performed using the distance restraints from the automatic NOE assignment protocol within CYANA 2.0 with minimal manual assignment to prevent user biasing (26–28). Error tolerances of 0.04 and 0.02 ppm were used, and 100 structures in each of eight CYANA iterations were calculated using 10,000 torsion angle dynamics steps per structure. RMSDs were calculated over residues 3–74 of VhACP during the CYANA runs, corresponding to the well structured region of the protein. The 30 lowest energy structures were kept in each iteration and used for statistical analysis. In the final structure calculation, upper and lower hydrogen bond distance restraint tables were added based on the helical regions observed in the previous VhACP structure calculation. The programs MOLMOL (29) and VMD (30) were used to visualize the PDB coordinates and to generate figures of various ACP structures.
Circular Dichroism Spectroscopy
Following purification, the proteins were transferred to 5 mm Ca2+-free Hepes buffer, pH 7, and 1 mm DTT was added to prevent protein dimerization. The CD spectra were obtained on a Jasco J-810 spectropolarimeter in a 0.1 cm quartz cell at room temperature. The spectra were recorded from 260 to 195 nm at a slow scanning speed. The protein concentration was 10 μm, and experiments were performed three times, each composed of 15 accumulations. To assay the protein conformation in the absence of divalent cations, 0.1 mm EDTA was added to the samples (18). The CD spectra of magnesium or calcium ACP were collected in the presence of 10 mm MgCl2 or 2–20 mm CaCl2.
Differential Scanning Calorimetry
The DSC experiments were carried out in a VP-DSC microcalorimeter. The protein was dissolved in 5 mm Hepes, pH 6.5 to a concentration of 30 μm and tested either in the presence or absence of 2 mm CaCl2. To prevent protein dimerization, β-mercaptoethanol (1 mm) was added, which causes fewer baseline distortions than DTT. The Ca2+-free sample was prepared by running the protein through a BAPTA calcium sponge column (Invitrogen). Lower calcium concentrations were used to ensure minimal baseline interference and should affect the structure in the same fashion as the higher calcium concentrations (18). Each protein sample was loaded into the DSC immediately after two consecutive buffer-only heating cycles, which account for thermal memory compensation of the DSC calorimeter and baseline correction, respectively. The proteins were heated from 10 to 100 °C at a scan rate of 60 K/h with a filter period of 16 ps. The pressure was kept above 28 psi to keep the sample stable at higher temperatures. The samples were cooled and rescanned to check for reversibility.
RESULTS
Solution Structure
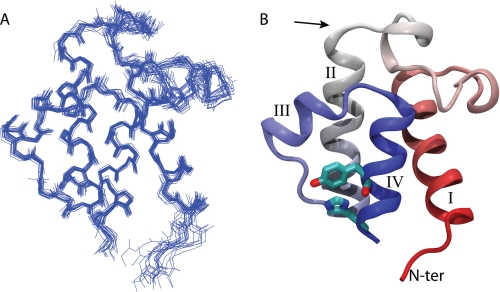
The NMR solution structure of VhACP A75H possesses four α-helices including three helices running almost parallel to each other and surrounding the hydrophobic core of ACP (Fig. 2). The helices are well defined, and the structures converged to a backbone RMSD of 0.48 Å (res. 1–74) (Table 1). Helix III is shorter than the other helices and runs across the long axis of ACP between helices II and IV. This secondary structure pattern is highly conserved among all ACP forms (3). Helices I-IV span residues 4–15, 36–50, 56–60, and 65–75, respectively, as determined by the program STRIDE (Fig. 2) (31). Ser-36, the prosthetic group attachment site, resides at the start of helix II, which is the norm in ACP structures. The binding pocket of ACP is lined with hydrophobic residues and is closed off to the solvent similar to other holo-ACP structures. A small cavity remains in the center of ACP toward helix I, which has previously been described as subpocket II (32). The structure of VhACP A75H closely resembles EcACP (PDBID 1L0H), with a backbone RMSD of 1.40 Å over residues 3–74 and superposition of the two structures shows virtually identical secondary structure. The residues that differ in primary structure between the E. coli and V. harveyi proteins (Fig. 1) are all solvent exposed with the exception of Val-72 in V. harveyi (Ile-72 in E. coli). This residue is buried at the bottom of the cavity in both proteins but does not alter the protein structure significantly. The mutated residue His-75 is located at the C-terminal end of helix IV. It is involved in a parallel stacking interaction with Tyr-71, which precedes His-75 by one α-helical turn (Fig. 2B). This stacked conformation is also observed in the EcACP structure (6) and positions the residues optimally for cation-pi interactions (33) and is consistent with the observation that His-75 in EcACP has an elevated pKa between 7.5 and 8.0 (34).
FIGURE 2.
NMR solution structure of VhACP A75H. A shows the backbone overlay of the 20 lowest energy structures of VhACP overlaid on the four α-helices, shown in the same orientation as B. B, shows a ribbon diagram of the lowest energy structure, colored in a spectrum from red to blue from the N to the C terminus, respectively. His-75 and Tyr-71 are shown in stick representation to highlight their stacking interaction. The arrow shows the position of Ser-36.
TABLE 1.
Statistics of NMR solution structure of V. harveyi ACP A75H
| NOE crosspeaks | |
| Selected | 1930 |
| With assignment | 1859 |
| Hydrogen bond restraints | 56 |
| Distance restraint violations (>0 Å) | 2 |
| Upper distance limits | |
| Total | 1553 |
| Short-range 1< i-j | 781 |
| Medium-range 1 < i-j < 5 | 405 |
| Long i-j > = 5 | 367 |
| PROCHECK Ramachandran analysis (%) | |
| In most favored region | 78.6 |
| In additionally favored region | 21 |
| In generously allowed region | 0 |
| In disallowed regions | 0.3 |
| Coordinate precision of folded region (residues 3–74; Å) | |
| Backbone atoms | 0.48 ± 0.09 |
| Heavy atoms | 0.97 ± 0.09 |
| Cyana target function | 0.6 ± 0.1 |
NMR Studies of Divalent Cation Binding to ACP
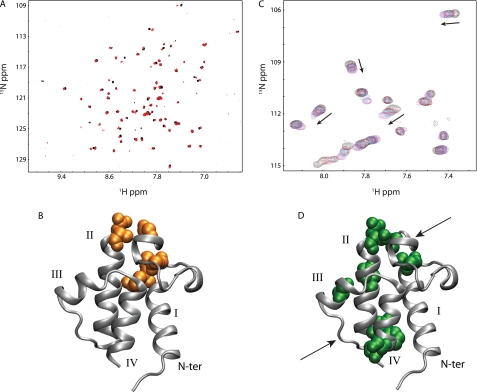
Preliminary HSQC NMR experiments of the wild-type VhACP protein showed that the calcium-free protein is unfolded, while addition of Ca2+ or Mg2+ induced protein folding (not shown). The HSQCs of Ca2+ and Mg2+ bound ACP show very similar profiles and only four residues have Δδave > 0.05ppm, which are Gln-14, Leu-15, Gly-33, and Asp-35 (Fig. 3). Plotted on the VhACP A75H structure, it can be seen that these residues reside close in space to each other, Gln-14 and Leu-15 at the C-terminal end of helix I and Gly-33 and Asp-35 adjacent, just N-terminal of helix II (Fig. 3B). Therefore, it appears that there are only minor differences between the Ca2+- and Mg2+-bound ACP forms, which allow us to readily compare results obtained with either divalent cation.
FIGURE 3.
Divalent cation binding to wild-type and A75H V. harveyi ACP. A, natural abundance HSQC spectra of wild-type V. harveyi ACP in the presence of calcium (red) and magnesium (black) ions suggest ACP adopts similar conformations in the presence of either divalent cation. B, VhACP A75H structure showing in orange where the residues display the largest chemical shift differences in the presence of the two cations. C, HSQC spectra of VhACP A75H collected at 0, 0.3, 0.5, 0.9, and 4.0 mm CaCl2 (black, red, blue, cyan, magenta, respectively) indicate that calcium binding occurs on the fast NMR timescale for both sites (see arrows). Only a portion of the HSQCs are shown for clarity. D, residues affected the most upon addition of calcium are highlighted in green, spatially matching the E. coli ACP divalent cation-binding sites (arrows).
One-dimensional proton NMR titrations of calcium and magnesium to wild-type VhACP are consistent with the presence of two calcium or magnesium-binding sites that display different binding modes, one in slow exchange and one in fast exchange on the NMR timescale (supplemental Fig. S1). To distinguish which binding mode occurs at which site, we employed the binding site elimination mutants A and B of VhACP (refer to Fig. 1) (18) to investigate calcium binding to each site independently. Consistent with the wild-type VhACP titrations, two different calcium-binding modes were observed for the site A and B mutants utilizing 1D proton NMR. The site A elimination mutant abolishes divalent cation binding at site A, which allows us to observe isolated calcium binding at site B (18). Similarly, the reverse is true for the site B mutant (18). Calcium titration of the site A triple mutant displayed fast-exchange on the proton NMR timescale, indicative of calcium binding to site B (supplemental Fig. S1). In contrast, calcium titration of the site B mutant resulted in slow exchange on the NMR timescale, reflective of calcium binding properties at site A (supplemental Fig. S1). However in the wild-type VhACP protein titration, changes at sites A and B seem to occur simultaneously (supplemental Fig. S1), suggesting that both sites have a similar affinity for calcium. In addition, the mutant VhACP A75H only displays changes on the fast NMR timescale (see below). Taken together, this pattern suggests that the slow-exchange behavior of site A likely reflects the (slower) rate of protein (un)folding rather than the off-rate for the calcium ions.
To investigate the effect of the A75H mutation on the divalent cation binding properties of VhACP, further calcium titrations were performed on 15N-labeled protein (0.3 mm), visualizing the binding process using HSQC NMR experiments. The spectrum at 0 mm Ca2+ displays a well folded protein; however, several peaks of lower intensity are also seen in the region of the spectrum that is typical for unfolded proteins. Upon addition of 0.5 mm Ca2+, all of these lower intensity peaks disappear and only the peaks for folded VhACP A75H remain. Incremental additions of calcium up to 4 mm reveal that calcium binding to the folded VhACP A75H mutant protein occurs exclusively in fast exchange on the NMR timescale, unlike what is observed for wild-type VhACP (Fig. 3C). Residues that are affected by Δδave > 0.05ppm upon Ca2+ binding are Leu-15, Ala-34, Asp-35, Glu-41, Glu-49, Phe-50, Ala-59, and Ser-74. These residues lie either in close proximity to calcium-binding sites A or B (Fig. 3D). These results complement the data obtained by paramagnetic relaxation perturbation measurements for Mn2+ binding to EcACP (11).
Hydrogen-Deuterium Exchange
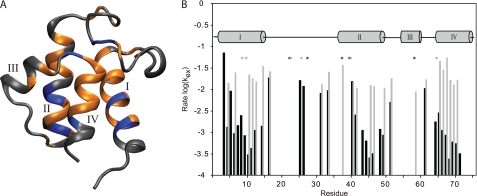
15N NMR relaxation experiments of calcium free and bound ACP reveal that there are no significant differences in dynamics on a ps-ns timescale (supplemental Fig. S2 and text). To assess whether there are any dynamic differences on a much slower timescale between VhACP A75H with and without calcium or magnesium, we performed H/D exchange studies. The first HSQC spectra collected were run after 10 min of sample preparation and showed significant differences between the two samples. VhACP A75H with 5 mm CaCl2 had 40 remaining peaks in the HSQC spectrum, while there were only 30 peaks remaining in the sample without any calcium present. The protected residues remaining in these spectra map to similar regions of VhACP A75H (Fig. 4). They encompass larger portions of helices I, II, and IV, the loop I portion adjacent to helix IV, and two residues in helix III. The residues that are seen in the first spectrum of the Ca2+ sample but not in the absence of calcium are Glu-4, Arg-6, Lys-8, Ser-27, Phe-28, Met-44, Ala-45, Glu-49, Thr-64, and Val-72 (Fig. 4). After ∼1.75 h, the HSQC spectrum of the calcium free sample only displays 2 peaks corresponding to residues Glu-5 and Leu-42, while the corresponding spectrum of the Ca2+-containing sample still displays 32 crosspeaks (Fig. 4B). After 2 h, the calcium-free protein spectrum does not contain any remaining peaks, whereas the calcium-bound protein spectrum at this point still contains 32 peaks. Even after 2 days, the calcium saturated VhACP A75H spectrum still shows several crosspeaks, corresponding to Ile-11, Val-12, Leu-46, and Ile-69. HSQC spectra collected 6 days after dissolving the lyophilized calcium saturated protein no longer show these peaks.
FIGURE 4.
A, residues observed after 30 min of H/D exchange in calcium-free VhACP A75H are shown in gold, while additional residues observed in the Ca2+-bound protein are shown in blue. B, H/D exchange rates as observed in Ca2+-bound (black) and Ca2+-free (gray) VhACP A75H, with lower values indicative of slower exchange. Note the vastly slower rates of the Ca2+-bound protein in the helical regions. Residues denoted by stars indicate peaks were present in too few spectra to reliably determine any rates. Binding of Mg2+ has a similar effect on the H/D exchange rates as Ca2+ (not shown).
pH Titrations
To study the pH induced protein expansion and investigate the role of the positive charge of His75, we collected HSQC spectra at increasing pH values from 7.0 to 10.0 in the absence of divalent cations. At pH 7.5, the majority of the changes occur in the far C terminus of ACP, around His-75 and also at Ile-54, which lies in loop II (supplemental Fig. S3). At pH 8.0 and 8.5, the chemical shift changes in the C terminus increase in magnitude and the far N terminus shows larger perturbations as well. The magnitude of the chemical shift changes appears to essentially level off at pH 8.5 (supplemental Fig. S3). In addition to the changes in chemical shifts, many of the peaks disappear as the pH increases. At pH 7.0, 76 out of 80 possible amide peaks were present, representing 76 out of 80 amino acids in the protein. This number decreases to 69, 67, 61, 27, and 0 peaks at pH 7.5, 8.0, 8.5, 9.0, 9.5, and 10, respectively.
CD Spectroscopy
The secondary structure of various ACP forms was monitored using circular dichroism experiments. Position 75 of ACP is of particular interest in order to understand how the VhACP A75H mutation is able to stabilize the folded conformation of this protein. To that extent, we also analyzed VhACP mutants Y71W and A75W as well as EcACP H75A and compared them to the wild-type proteins (supplemental Fig. S4). Similar to wild-type VhACP in the absence of divalent cations, the Y71W and A75W mutants display CD spectra that are characteristic of being largely unfolded. Upon addition of either 10 mm MgCl2 or CaCl2, the proteins adopt α-helical secondary structure as revealed by characteristic double dips at 220 and 208 nm in the CD spectra. To test whether His-75 in EcACP is solely responsible for the stability of the protein in the absence of stabilizing divalent cations, the EcACP mutant H75A was also assessed. The CD spectra of this protein are clearly distinct from wild-type EcACP as they display little secondary structure (supplemental Fig. S4). Upon addition of MgCl2, the CD spectrum of EcACP H75A returns to that characteristic of the folded conformation of ACP with high α-helical content. The level of secondary structure destabilization introduced by the H75A mutation in EcACP is not as pronounced as in wild-type VhACP, but it is clearly lower than in wild-type EcACP. The intensity ratio of the CD spectra at 220 nm with and without divalent cations measures 2.3 for EcACP H75A. This is intermediate to that of wild-type EcACP and VhACP, which measure 1.0 ± 0.02 and 6.2 ± 0.32, respectively. The VhACP mutants Y71W and A75W display slightly more α-helical character in the divalent cation-free state than the wild-type protein, with ratios of 3.8 and 4.4, respectively.
Differential Scanning Calorimetry
Wild-type E. coli and V. harveyi ACP, as well as VhACP A75H were studied using DSC experiments. DSC is a conformationally sensitive biophysical technique that allows for the determination of protein melting temperatures which reflect the thermal stability of the proteins being investigated. The calcium-free state yielded lower melting temperatures compared with the calcium-bound state in all proteins studied, which is in agreement with previous DSC studies of EcACP (35). Wild-type VhACP is unfolded, and it displayed no substantial transitions in the absence of calcium; it has a Tm of 66.4 °C upon addition of this divalent cation. The VhACP A75H mutant displayed a Tm of 48.8 °C, which is raised to 72.0 °C in the presence of calcium. The Tm for the VhACP A75H mutant protein in the presence of Mg2+ is 69.4 °C, which is very close to the value obtained with Ca2+. The wild-type EcACP has slightly higher Tm values of 52.7 °C and 73.3 °C in the absence and presence of calcium, respectively.
DISCUSSION
NMR Solution Structure of V. harveyi ACP A75H
The solution structure of VhACP A75H is similar to ACPs from other organisms that have been structurally characterized and this structural similarity is likely the reason why some of these ACPs are functionally interchangeable (36). The NMR structures of E. coli apo-, holo-, and butyryl-ACP show that helix III moves away from helix II and it is suggested that this is based on the nature of the attachment to Ser-36 (37). The VhACP A75H structure fits with this model, because helix III is oriented similarly as in the E. coli apo- and holo-ACP structures. In contrast to the type I rat ACP, the C-terminal end of loop I of VhACP is located toward helix I and therefore does not block the cavity entrance (38). This is consistent with the hypothesis that this portion of loop I rests away from the cavity entrance in ACPs that are capable of binding acyl chains in the substrate pocket, because VhACP is believed to do so (12). The hydrophobic cavity is closed off from the solvent in VhACP A75H, but a small hydrophobic pocket still exists inside the protein where the acyl chains are accommodated. Interestingly, this internal cavity is directed toward helix I of ACP, rather than pointing down along helix II, which is the conformation observed in the E. coli acyl-ACP structures (6, 7). Instead, the cavity observed in the VhACP A75H structure matches subpocket II of ACP that was recently described in molecular dynamics simulations (32). Evidence of subpocket II was also provided by NMR studies of P. falciparum ACP, and it appears likely that the acyl chains spend time in both subpockets in vivo (32, 39). Further experiments are needed to determine how this influences acyl chain binding and whether potential inhibitors can be optimized based on the binding to both subcavities.
Unique Folding of V. harveyi ACP
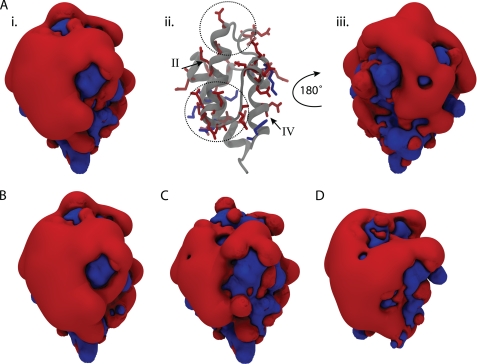
VhACP has an overall negative charge of −17, with 22 anionic residues and only 5 cationic residues (Fig. 1). The electrostatic surface plot of VhACP reveals a polarized landscape with one side of the protein exclusively composed of anionic charges while the opposite side is more interspersed with cationic residues (Fig. 5). The anionic face of VhACP surrounds helix II, which encompasses the two divalent cation-binding sites A and B that are located at the N- and C-terminal ends of the helix, respectively (Figs. 1 and 5). The high concentration of anionic charges results in a large electronegative field that protrudes from that portion of VhACP and thereby to strong electrostatic repulsion (Fig. 5). The clashing charges contribute to the destabilization of wild-type VhACP, which can be alleviated by either divalent cation binding or the introduction of cationic residues in the protein (40). This is precisely what occurs in VhACP A75H, where the introduction of a cationic residue neutralizes some of the tension in this region (Fig. 5, A and B). Nevertheless, there is still a large anionic domain present in VhACP A75H (Fig. 5A). This is perhaps not surprising, because wild-type EcACP also displays a large anionic lobe (Fig. 5D) but still folds properly at neutral pH in the absence of divalent cations. To investigate the effect of divalent cation binding on the VhACP surface profile, we modified the coordinates of VhACP A75H to reflect the charge state when bound by two Ca2+ or Mg2+ ions by introducing two +2 charges at their binding sites. In this scenario, the anionic domain is significantly reduced over the wild-type protein (Fig. 5B versus C), although even then a sizeable anionic side is seen. It should be noted that divalent cation binding not only stabilizes ACP by alleviating charge repulsion, but additionally through coordination of the metal ions, which result in favorable enthalpic contributions. The changes in the size of the anionic domain of ACP correlate with the changes in melting temperatures measured by DSC. That is, decreasing the size of the anionic domain of ACP corresponds to elevated melting temperatures, supporting the important role of charge repulsion in its destabilization. The VhACP E41K mutant also supports this idea, as this mutation in the center of helix II removes the dependence on divalent cations for proper folding (40).
FIGURE 5.
Electrostatic potential plots of various ACPs. Isocontour surfaces are shown at ± 3 kT/e, with the blue surface being cationic, while the red shows the anionic surface. VhACP A75H is shown in A, with the surface plot in the same orientation in i as in the ribbon diagram in ii and rotated about the y axis by 180° in iii. Aii highlights the position of charged residues on the structure of ACP with helices II and IV labeled with roman numerals. The divalent cation-binding sites A and B are highlighted by the upper and lower dotted circles, respectively. B shows the surface of wild-type VhACP, while C shows ACP A75H with two +2 charges placed at the calcium-binding sites. D displays the electrostatic surface plot of wild-type EcACP. B–D are oriented the same as in Ai.
Our pH titration studies on VhACP A75H lend further credence to the importance of helix II in the destabilization of VhACP. At elevated pH values, the number of peaks observed in the HSQC spectra gradually decreases. This can either occur because of local unfolding of ACP, which leads to a molten globule-like conformation and NMR chemical exchange processes that broaden the signals or secondly, due to an increase in the hydrogen exchange rate that takes place at elevated pH. Considering the well documented unfolding of ACP that occurs at higher pH (12, 21, 41), protein unfolding is likely to play the determining role in the disappearance of the signals in the HSQC spectra. Notably, none of the residues in helix II display any signal at pH 9.5, with the exception of Glu-48 at the C-terminal end of helix II. This suggests that the anionic region surrounding helix II is the least stable region of ACP, in agreement with the electrostatic plots presented earlier.
As mentioned above, cation-pi interactions can contribute to the stabilization of VhACP A75H as well. The ring of His-75 is positioned in a parallel, stacked orientation relative to Tyr-71 in helix IV, which is optimal for cation-pi interactions (Fig. 2B) (33). To investigate whether a pi-pi stacking interaction with Tyr-71 is capable of abolishing VhACP dependence on divalent cations, we replaced His-75 with a tryptophan residue. The indole ring of tryptophan contains a large pi-electron system that should be suitable for pi-pi stacking interactions with Tyr-71. However, our CD studies show that Trp-75 does not sufficiently stabilize the protein to maintain the folded conformation under neutral conditions (supplemental Fig. S4). The substitution of Tyr by Trp in the Y71W mutant protein did not completely stabilize the overall fold of VhACP either. We therefore conclude that the solvent exposed stacking interactions between the aromatic Tyr and His side chains are necessary for stabilizing the folding of helix IV, and as a result the overall protein. The location of His-75 close to the C-terminal end of helix IV may further contribute to the stabilization of ACP. Because of the helix dipole, the positively charged side chain of His-75 will interact favorably with the anionic end of the helix dipole.
Because it was identified that a single mutation to wild-type VhACP is able to dramatically change its folding behavior, we also sought to evaluate the role of His-75 on the stability of wild-type EcACP. Our CD results of EcACP H75A indicate that this residue is similarly important for the stability of EcACP as it is for VhACP (supplemental Fig. S4). The amount of destabilization is not as high as it is for VhACP, as the intensity ratio at 220 nm of our CD studies shows. This may be because EcACP is overall less anionic than VhACP, which decreases the electrostatic repulsion in the anionic lobe (Figs. 1 and 5). The presence of a His residue in the far C-terminal region of ACP is quite rare among ACPs from various organisms and frequently one or more Lys residues are found instead (3). Interestingly, H. pylori ACP possesses a Lys residue at the C terminus, but it was still reported to be partially unfolded at neutral pH (16). Its overall charge is −14, which is equal to EcACP and less anionic than VhACP (overall charge −17). It is possible that the complete absence of cationic charges in the N terminus is responsible for the destabilization of this protein instead (Fig. 1).
Divalent Cation Binding to ACP
It was shown previously that EcACP has the ability to bind divalent cations and that Mn2+, as a substitute for Ca2+ and Mg2+, is bound at two separate sites around helix II (11). These three divalent metal ions bind with similar affinity to EcACP (34). Furthermore, divalent cation binding stabilizes FA synthase ACPs and protects these proteins against alkaline induced unfolding (41). Interestingly, this is not the case for at least one ACP analog involved in lipoteichoic acid synthesis, the d-alanyl carrier protein, which does not appear to bind calcium at all (42). The HSQC spectra of wild-type VhACP indicate that Mg2+ and Ca2+ binding have the same effect on the structure. This is in contrast to other divalent cation-binding proteins that utilize EF-hand calcium-binding motifs as these display significantly different binding modes depending on the ion bound (43).
Our dynamics experiments provide further insights on the effect of divalent cations on VhACP A75H. The 15N relaxation data indicate that the motions of calcium-free and -bound states are highly similar on a ps-ns timescale (supplemental Fig. S2). On the other hand, the H/D exchange experiments display notable differences in protein behavior, indicating that the dynamics of ACP on a much slower timescale are highly affected by divalent cations. The data obtained by the H/D exchange experiments correlate well with the denaturation temperatures obtained by DSC. The protected regions in the H/D experiments closely correlate to the more rigid portions in the 15N dynamics experiments, and, on the other hand, loops I and II are among the most flexible portions on both timescales. Interestingly, the presence of Ca2+ or Mg2+ does not alter the regions of stability of ACP, but there seems to rather be an overall stabilization of the protein. This has been described for the protein calbindin D9K as well, where an overall stabilization of hydrogen-bonded residues was observed, not only those involved in calcium binding (44).
Biological Implications
The highly anionic character of VhACP and its poor stability have led to its categorization as a natively unfolded protein (3). Typical for these types of proteins, it was shown that enzyme binding can induce protein folding as well (17). In vivo, this effect may be less pronounced considering that there are basal levels of magnesium (1–2 mm) (45) present that would keep VhACP in its folded state. Nevertheless, the unfolded state that is probed in vitro is a reflection of the inherent flexibility that is present in the protein in vivo and contributes to ACP functionality. For example, its dynamic nature is thought to be important for interactions with the large number of partner enzymes and broad range of bound acyl chains (3). A single, rigid conformation would be too limiting in regards to which binding partners can access ACP and acyl chain extraction would be more difficult. Therefore, the unfolded conformation can be considered a quasi extreme condition of the highly dynamic protein present in vivo. Considering the proximity of the divalent cation-binding sites to the phosphopantetheine attachment site and the acyl chain-binding pocket, it is worthwhile to consider the effect of ion binding on the behavior of the prosthetic arm. The arm is attached at Ser-36, which lies central to metal ion-binding site A (11). Site B lies at the C-terminal end of helix II and also encompasses parts of loop II, which makes up a portion of the hydrophobic binding pocket. It is therefore not unexpected that the divalent cation binding affinity is different in apo-, holo-, and acyl-ACP (18, 34). It has recently been suggested that the electrostatic repulsion between helices II and III of P. falciparum ACP is important for the prompt opening and extraction of the acyl chain (39). Therefore, divalent cation binding will likely affect this process by diminishing the repulsive forces between the two helices (Fig. 5). As such, divalent cation binding may have consequences on the acyl chain extraction process, which would affect substrate delivery of the acyl chain to partner enzymes. Slight differences in dynamics are in fact observed in the region surrounding helix III in the 15N relaxation experiments, and the H/D exchange is slowed in this region as well in the presence of calcium. Furthermore, it was recently described that acyl chains of different lengths bound to spinach ACP affect its dynamics, illustrating that there is a correlation between protein dynamics and the substrates bound to ACP (46).
Electrostatic interactions, in particular the highly anionic recognition helix II, are important in ACP:enzyme interactions (9, 18). Considering the proximity of the divalent cation-binding sites to helix II of ACP, it is not surprising that these cations affect the interactions between ACP and the FAS enzymes. It has been shown that FAS activity is highest in the presence of 5–10 mm MgCl2, and it is believed that this effect is due to ionic interactions with ACP (47). Therefore, divalent cation binding should be considered when studying the electrostatic surface of ACP. Subtle changes on the surface of ACP are thought to be important for the recognition of specific intermediate forms (e.g. β-keto-, β-hydroxyl, trans-2-enoyl-groups) of ACP by appropriate enzymes (48). These intermediates are believed to convey which binding partner is present through the exposure of single hydroxyl or ketone groups that are specifically recognized by FAS enzymes (48). It is therefore important to comprehend how precisely divalent cations affect the conformation of ACP, such that a better understanding may be achieved of which factors govern ACP recognition by FAS enzymes.
Supplementary Material
Acknowledgments
We thank Dr. A. Yamniuk for help with the biophysical characterization of ACP and Dr. H. Ishida and Zhihong Liu for help with the 15N relaxation experiments.
This work was supported by operating grants from the Natural Sciences and Engineering Research Council of Canada (to D. M. B.) and the Canadian Institute of Health Research (to H. J. V.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S4 and data.
The atomic coordinates and structure factors (code 2L0Q) have been deposited in the Protein Data Bank, Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org/).
- ACP
- acyl carrier protein
- EcACP
- E. coli ACP
- FA
- fatty acid
- FAS
- FA synthase
- NMR
- nuclear magnetic resonance
- VhACP
- V. harveyi ACP
- RMSD
- root mean square deviation
- PDB
- Protein Data Bank.
REFERENCES
- 1.Maier T., Jenni S., Ban N. (2006) Science 311, 1258–1262 [DOI] [PubMed] [Google Scholar]
- 2.Chan D. I., Vogel H. J. (2010) Biochem. J. 430, 1–19 [DOI] [PubMed] [Google Scholar]
- 3.Byers D. M., Gong H. (2007) Biochem. Cell Biol. 85, 649–662 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y. M., Rock C. O. (2008) Nat. Rev. Microbiol. 6, 222–233 [DOI] [PubMed] [Google Scholar]
- 5.White S. W., Zheng J., Zhang Y. M., Rock C. O. (2005) Annu. Rev. Biochem. 74, 791–831 [DOI] [PubMed] [Google Scholar]
- 6.Roujeinikova A., Baldock C., Simon W. J., Gilroy J., Baker P. J., Stuitje A. R., Rice D. W., Slabas A. R., Rafferty J. B. (2002) Structure 10, 825–835 [DOI] [PubMed] [Google Scholar]
- 7.Roujeinikova A., Simon W. J., Gilroy J., Rice D. W., Rafferty J. B., Slabas A. R. (2007) J. Mol. Biol. 365, 135–145 [DOI] [PubMed] [Google Scholar]
- 8.Zornetzer G. A., Fox B. G., Markley J. L. (2006) Biochemistry 45, 5217–5227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y. M., Marrakchi H., White S. W., Rock C. O. (2003) J. Lipid Res. 44, 1–10 [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y. M., Rao M. S., Heath R. J., Price A. C., Olson A. J., Rock C. O., White S. W. (2001) J. Biol. Chem. 276, 8231–8238 [DOI] [PubMed] [Google Scholar]
- 11.Frederick A. F., Kay L. E., Prestegard J. H. (1988) FEBS Lett. 238, 43–48 [DOI] [PubMed] [Google Scholar]
- 12.de la Roche M. A., Shen Z., Byers D. M. (1997) Arch. Biochem. Biophys. 344, 159–164 [DOI] [PubMed] [Google Scholar]
- 13.Byers D. M., Meighen E. A. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 6085–6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flaman A. S., Chen J. M., Van Iderstine S. C., Byers D. M. (2001) J. Biol. Chem. 276, 35934–35939 [DOI] [PubMed] [Google Scholar]
- 15.Keating M. M., Gong H., Byers D. M. (2002) Biochim. Biophys. Acta 1601, 208–214 [DOI] [PubMed] [Google Scholar]
- 16.Park S. J., Kim J. S., Son W. S., Lee B. J. (2004) J. Biochem. 135, 337–346 [DOI] [PubMed] [Google Scholar]
- 17.Gong H., Murphy P. W., Langille G. M., Minielly S. J., Murphy A., McMaster C. R., Byers D. M. (2008) Biochim. Biophys. Acta 1784, 1835–1843 [DOI] [PubMed] [Google Scholar]
- 18.Gong H., Murphy A., McMaster C. R., Byers D. M. (2007) J. Biol. Chem. 282, 4494–4503 [DOI] [PubMed] [Google Scholar]
- 19.Keating D. H., Carey M. R., Cronan J. E., Jr. (1995) J. Biol. Chem. 270, 22229–22235 [DOI] [PubMed] [Google Scholar]
- 20.Lambalot R. H., Walsh C. T. (1997) Vitamins Coenzymes 279, 254–262 [DOI] [PubMed] [Google Scholar]
- 21.Rock C. O., Cronan J. E., Jr., Armitage I. M. (1981) J. Biol. Chem. 256, 2669–2674 [PubMed] [Google Scholar]
- 22.Hwang T. L., Shaka A. J. (1995) J. Magn. Reson. 112, 275–279 [Google Scholar]
- 23.Delaglio F., Grzesiek S., Vuister G. W., Zhu G., Pfeifer J., Bax A. (1995) J. Biomol. NMR 6, 277–293 [DOI] [PubMed] [Google Scholar]
- 24.Johnson B. A., Blevins R. A. (1994) J. Biomol. NMR 4, 603–614 [DOI] [PubMed] [Google Scholar]
- 25.Wüthrich K. (1986) NMR of Proteins and Nucleic Acids, John Wiley & Sons, New York [Google Scholar]
- 26.Güntert P., Braun W., Wüthrich K. (1991) J. Mol. Biol. 217, 517–530 [DOI] [PubMed] [Google Scholar]
- 27.Güntert P., Mumenthaler C., Wüthrich K. (1997) J. Mol. Biol. 273, 283–298 [DOI] [PubMed] [Google Scholar]
- 28.Herrmann T., Güntert P., Wüthrich K. (2002) J. Mol. Biol. 319, 209–227 [DOI] [PubMed] [Google Scholar]
- 29.Koradi R., Billeter M., Wüthrich K. (1996) J. Mol. Graph. 14, 51–55 [DOI] [PubMed] [Google Scholar]
- 30.Humphrey W., Dalke A., Schulten K. (1996) J. Mol. Graph. 14, 33–38 [DOI] [PubMed] [Google Scholar]
- 31.Frishman D., Argos P. (1995) Proteins: Struct. Funct. Genet. 23, 566–579 [DOI] [PubMed] [Google Scholar]
- 32.Chan D. I., Stockner T., Tieleman D. P., Vogel H. J. (2008) J. Biol. Chem. 283, 33620–33629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma J. C., Dougherty D. A. (1997) Chem. Rev. 97, 1303–1324 [DOI] [PubMed] [Google Scholar]
- 34.Tener D. M., Mayo K. H. (1990) Eur. J. Biochem. 189, 559–565 [DOI] [PubMed] [Google Scholar]
- 35.Horvath L. A., Sturtevant J. M., Prestegard J. H. (1994) Protein Sci. 3, 103–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerra D. J., Dziewanowska K., Ohlrogge J. B., Beremand P. D. (1988) J. Biol. Chem. 263, 4386–4391 [PubMed] [Google Scholar]
- 37.Wu B. N., Zhang Y. M., Rock C. O., Zheng J. J. (2009) Protein Sci. 18, 240–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ploskoń E., Arthur C. J., Evans S. E., Williams C., Crosby J., Simpson T. J., Crump M. P. (2008) J. Biol. Chem. 283, 518–528 [DOI] [PubMed] [Google Scholar]
- 39.Upadhyay S. K., Misra A., Srivastava R., Surolia N., Surolia A., Sundd M. (2009) J. Biol. Chem. 284, 22390–22400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong H., Byers D. M. (2003) Biochem. Biophys. Res. Commun. 302, 35–40 [DOI] [PubMed] [Google Scholar]
- 41.Schulz H. (1975) J. Biol. Chem. 250, 2299–2304 [PubMed] [Google Scholar]
- 42.Volkman B. F., Zhang Q., Debabov D. V., Rivera E., Kresheck G. C., Neuhaus F. C. (2001) Biochemistry 40, 7964–7972 [PubMed] [Google Scholar]
- 43.Gifford J. L., Walsh M. P., Vogel H. J. (2007) Biochem. J. 405, 199–221 [DOI] [PubMed] [Google Scholar]
- 44.Skelton N. J., Kördel J., Akke M., Chazin W. J. (1992) J. Mol. Biol. 227, 1100–1117 [DOI] [PubMed] [Google Scholar]
- 45.Alatossava T., Jütte H., Kuhn A., Kellenberger E. (1985) J. Bacteriol. 162, 413–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zornetzer G. A., Tanem J., Fox B. G., Markley J. L. (2010) Biochemistry 49, 470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulz H., Weeks G., Toomey R. E., Shapiro M., Wakil S. J. (1969) J. Biol. Chem. 244, 6577–6583 [PubMed] [Google Scholar]
- 48.Chan D. I., Tieleman D. P., Vogel H. J. (2010) Biochemistry 49, 2860–2868 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.