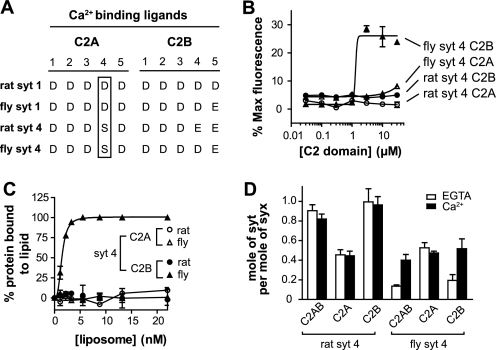
FIGURE 4.
The C2B domain of fly SYT4 directly promotes fusion in response to Ca2+. A, alignment of Ca2+ ligands in rat and fly Syt. The conserved Asp-to-Ser Ca2+ ligand substitution is indicated by an open rectangular box. B, fusion reactions were carried out as described in the legend for Fig. 1B, using isolated fly and rat SYT4 C2A or C2B domains. Normalized fluorescence signals were plotted against [C2 domain]. Fly SYT4 C2B triggered fusion in response to Ca2+; rat SYT4 C2A, C2B and fly SYT4 C2A all failed to stimulate fusion. C, the ability of isolated C2 domains from rat and fly SYT4 to bind PS-bearing liposomes was monitored using a co-sedimentation assay. The amount of bound protein was determined and plotted against [liposome]. Fly SYT4 C2B bound to PS-bearing liposomes in response to Ca2+; the other three C2 domains failed to exhibit significant levels of binding. D, the t-SNARE binding activities of the isolated C2 domains from rat and fly SYT4 were monitored using a co-flotation assay as described in the legend for Fig. 2B. The molar ratio of Syt to syntaxin in each sample was plotted. Both C2 domains of rat SYT4, as well as fly SYT4 C2A, bound to t-SNAREs in a Ca2+-independent manner. In contrast, fly SYT4 C2B bound to t-SNARE in a Ca2+-dependent manner (n = 3). For representative gels of these experiments, please see supplemental Fig. S3.