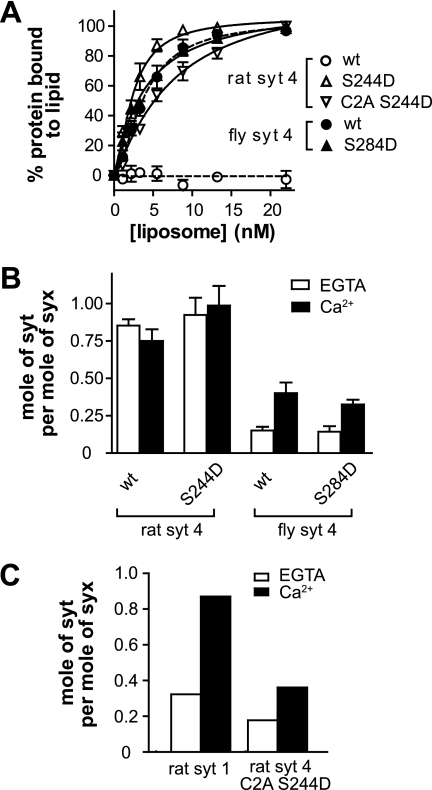
FIGURE 6.
PS and t-SNARE binding activities of rat and fly SYT4 Ser-to-Asp mutants. A, liposome binding assays were carried out by co-sedimentation and analyzed as described in the legend for Fig. 2A. Rat SYT4 failed to bind. Rat SYT4 S244D, wild type fly SYT4, and fly SYT4 S284D all bound to PS-bearing liposomes in response to Ca2+. B, the molar ratio of Syt to syntaxin, as determined from co-flotation assays (described in the legend for Fig. 2B), was plotted (n = 3). Wild type and S244D mutant rat SYT4 bound to t-SNAREs in a Ca2+-independent manner. In contrast, wild type fly SYT4 and the S284D mutant exhibited Ca2+-promoted t-SNARE binding activity. C, co-immunoprecipitation of Syt with t-SNARE heterodimers composed of full-length SNAP-25B and syntaxin 1A. The isolated C2A domain of rat SYT4 S244D bound to t-SNAREs in a Ca2+-dependent manner. Rat SYT1 was analyzed in parallel and served as a control. For representative gels of these experiments, please see supplemental Fig. S5.