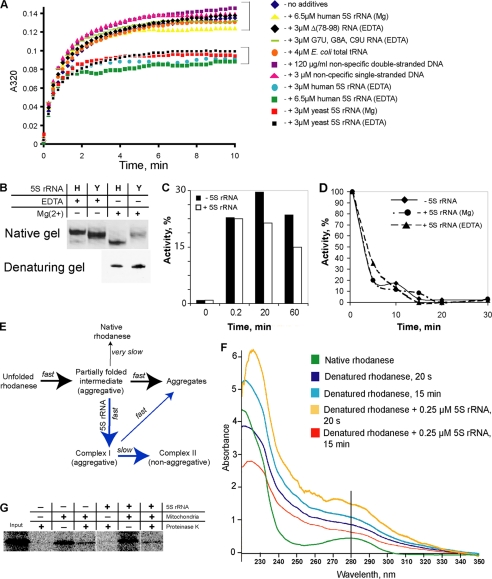
FIGURE 4.
Chaperone activity of 5 S rRNA upon its interaction with misfolded rhodanese. A, rhodanese aggregation assay in the presence of various nucleic acids. B, mobility of human (H) and yeast (Y) 5 S rRNAs in 8% 1× TBE native gel. 5 S rRNAs were folded either in the absence of magnesium (1 mm EDTA) or in the presence of 5 mm MgCl2. C, time course reactivation of urea-denatured rhodanese in the absence and in the presence of 3 μm human 5 S rRNAEDTA. D, thermal inactivation of rhodanese in the absence of 5 S rRNA and in the presence of 3 μm 5 S rRNAEDTA or 5 S rRNAMg. E, scheme describing behavior of urea-denatured rhodanese in a solution without denaturant (e.g. in the aggregation assay, see A) in the absence (black arrows) and in the presence (blue arrows) of human 5 S rRNAEDTA (developed from Refs. 17, 20). F, UV spectra of rhodanese (0.25 μg/μl) in native, urea-denatured, and 5 S rRNA-complexed forms, 20 s and 15 min after beginning of aggregation. G, import of rhodanese into isolated human mitochondria. 35S-Labeled proteins were synthesized in the cell-free PCR-optimized TnT system (Promega) either in the absence or in the presence of 4 μm human 5 S rRNAEDTA. Rhodanese does not demonstrate visible mobility shift upon import, because no cleavage of its mitochondrial transport signal occurs (24).