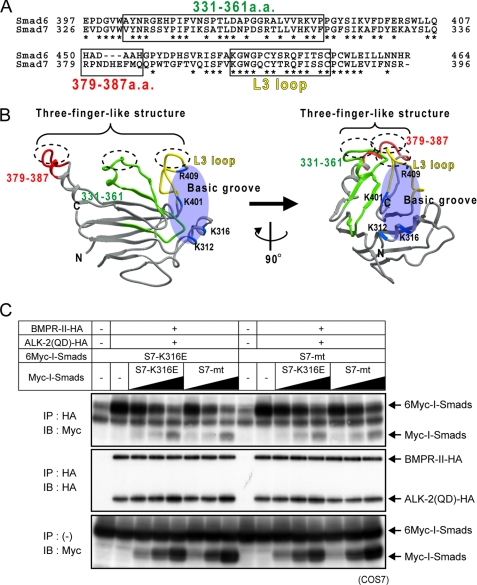
FIGURE 6.
Two distinct surfaces on Smad7 in interaction with type I receptors. A, alignment of amino acid sequences of Smad6 and Smad7. Residues involved in ALK-2 interaction are boxed, and identical residues in Smad6 and 7 are shown with asterisks. B, three-dimensional structural model of the MH2 domain of Smad7 based on the three-dimensional structure of Smad2 (PDB code 1DEV). Residues 331–361 are shown in green; residues 379–387, red; the L3 loop (residues 401–414), yellow; and the basic residues in the basic groove (Lys-312, Lys-316, Lys-401, and Arg-409), blue. C, competition of I-Smad mutants for interaction with ALK-2. COS7 cells were transfected with HA-tagged ALK-2, BMPRII, and Myc- or 6Myc-tagged I-Smad mutants. Receptors were immunoprecipitated (IP), and co-precipitated I-Smad mutants were visualized by immunoblotting (IB; top panel). Precipitated receptors (middle panel) and expression of I-Smad mutants (bottom panel) are also shown.