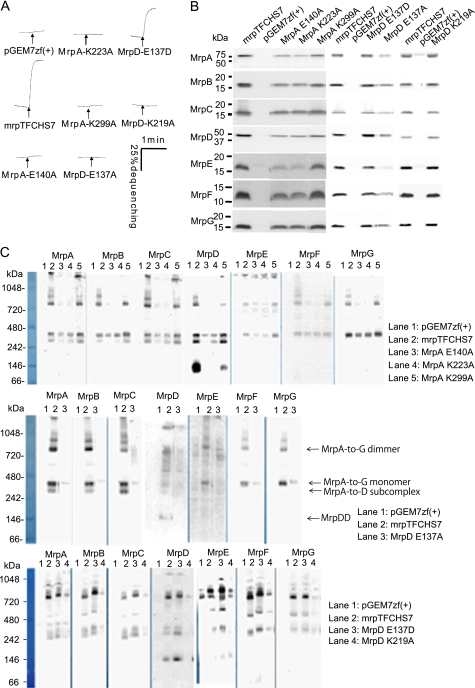
FIGURE 3.
Effects of mutations of charged amino acids of MrpA and MrpD, MrpA-Glu140, MrpA-Lys223, MrpA-Lys299, MrpD-Glu137, and MrpD-Lys219, that are conserved across MrpA, MrpD, and NADH-dehydrogenase-1 subunits NuoL/M/N. A, antiport activity is shown as the percentage of dequenching of acridine orange fluorescence when 10 mm Na+ was added (at the arrow) to energized everted vesicles that had achieved a steady-state pH, acid in, by respiratory proton pumping. Na+/H+ antiport activity was assayed at pH 8.5 in vesicles from transformants with the empty vector pGEM7zf(+) or the vector expressing full Mrp (pGEMmrpTFCHS7) or the mutant Mrp systems with the following point mutations: MrpA-E140A, -K223A, or -E137A or MrpD-E137A, -E137D, or -K219A. B, Western analyses of SDS-PAGE-fractionated membranes from the wild-type and the mutant transformants using antibodies against each Mrp subunit. The plasmid present in the membranes is indicated above each lane. C, detection of Mrp complexes by immunoblotting analysis of BN-PAGE. 30 μg of protein extracted from membrane was loaded onto gels for BN-PAGE fractionation and then transferred to PVDF membrane for immunoblotting. Mrp subunits were detected with antibodies against unique epitope tags linked to the C terminus of MrpA–MrpD and MrpG. MrpE and MrpF proteins were detected with polyclonal antibodies directed toward these proteins. Methodological details are described under “Experimental Procedures.”