Table 1.
CDR of 2 at −45 °C for 3 h using ligand 8 (or 9 where noted).
 | ||||
|---|---|---|---|---|
| Entry | E+ | Product(s) | % Yield | er |
| 1 | Me3SiCl | (S)-3 | 74 | 96:4 |
| 2 | Bu3SnCl | (S)-10 | 66 | 96:4 |
| 3 | Bu3SnCl | (R)-10 | 62 | 97:3a |
| 4 | CO2 | (R)-11 | 78 | 98:2 |
| 5 | MeOCOCl | (R)-12 | 88 | >99:1 |
| 6 | MeOCOCl | (S)-12 | 85 | >99:1a |
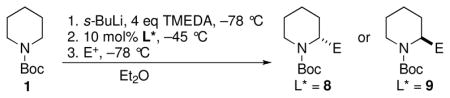
| 7 | PhNCO | (R)-13 | 68 | >99:1 |
| 8 | Allyl bromide | (R)-14 | 63 | 95:5b |
| 9 | Allyl bromide | (S)-14 | 59 | 96:4a,b |
| 10 | BnBr | (R)-15 | 65 | >99:1b |
| 11 | Cyclohexanone |
 (R)-16 |
60 | 94:6 |
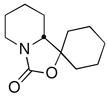
| 12 | PhCHOc |
 17 |
74 (62:38 dr) | >99:1 & 98:2 |
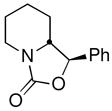
| 13 | 1-NpCHOc |
 18 |
66 (82:18 dr) | 94:6 & 93:7 |
| 14 | CH3CHOc |
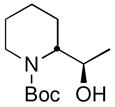 19 |
78 (85:15 dr) | >99:1 for both |
Using ligand 9
via Negishi cross coupling
Major diastereomer illustrated
