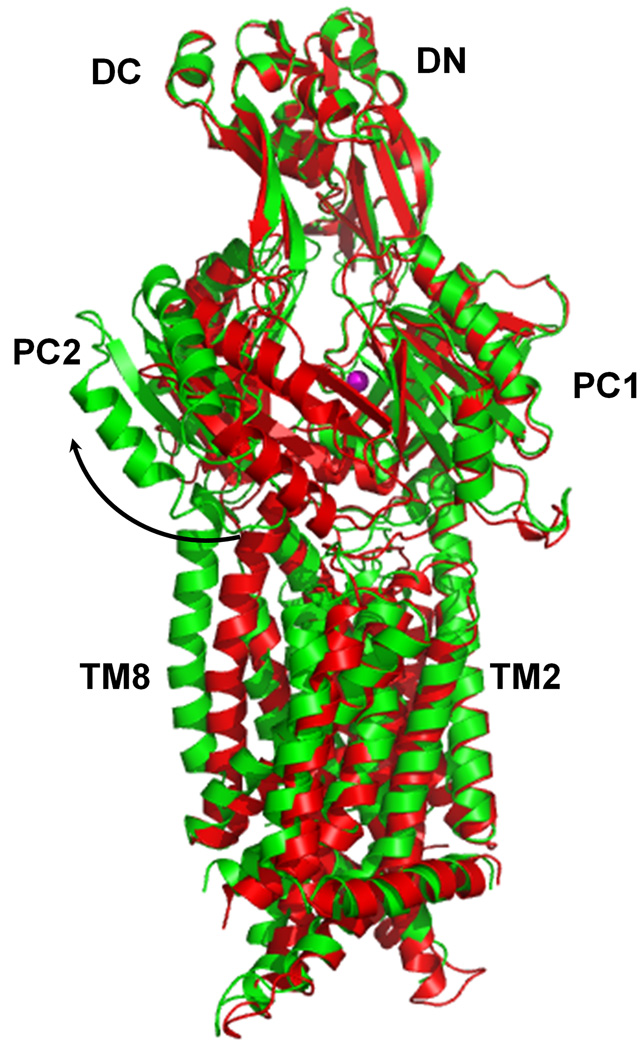
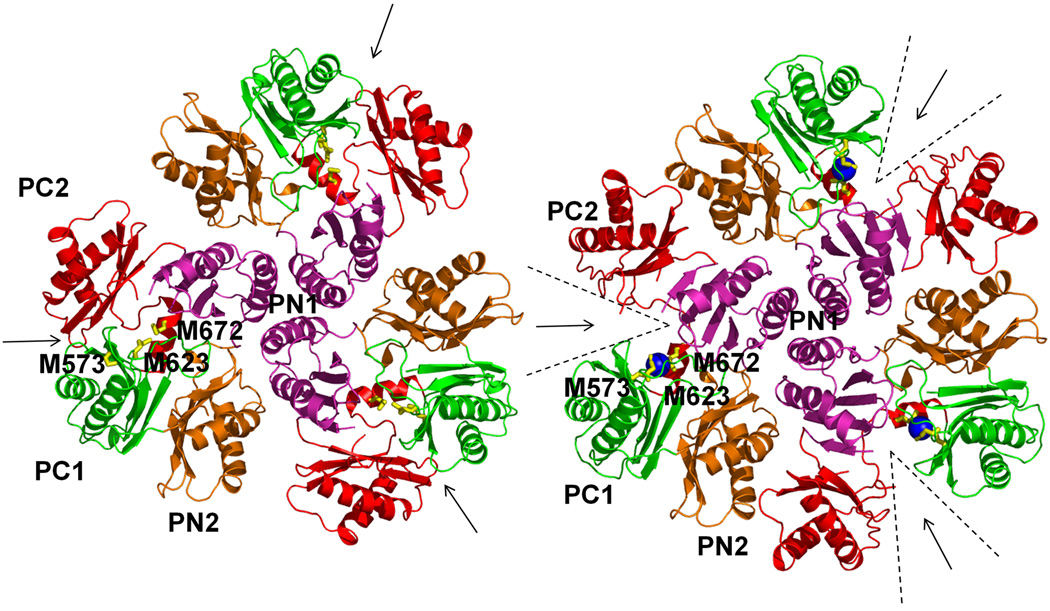
Figure 2.
Comparison of the apo and metal-bound structures of CusA. (a) Superposition of apo-CusA (red) onto CusA-Cu(I) (green). The bound Cu(I) is pink. Arrow indicates the shift of PC2 when comparing these two structures. (b) Conformational changes of the periplasmic domain of CusA. The conformation of each sub-domain of CusA before (left) and after (right) Cu(I) binding is shown. The cleft formed between PC1 and PC2 is opened after Cu(I) binding. This occurs by the horizontal helix moving to bind the ion, freeing TM8 to move and open. The bound coppers are blue. The arrows indicate the locations of the clefts.