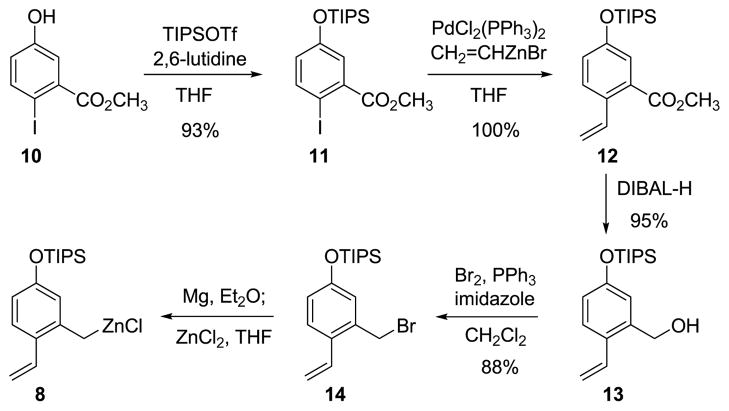
Figure 3. Preparation of the o-vinyl benzylzinc reagent 8.
The functionalized benzylzinc reagent 8 is prepared from the corresponding Grignard reagent and anhydrous zinc chloride. The Grignard reagent is readily formed from the benzyl bromide 14, which is prepared in multi-gram amounts by a four-step sequence. THF = tetrahydrofuran; Ph = phenyl; DIBAL-H = diisobutylaluminium hydride.