Abstract
Upon respiratory syncytial virus (RSV) challenge, mice previously immunized intramuscularly with inactivated whole virus express a Th2-like pattern of cytokine mRNA, while mice immunized with live virus intranasally express a Th1-like pattern. In this study, we evaluated the effects of anti-IL-4 treatment on the induction of immune responses after immunization. Mice treated with anti-IL-4 at the time of immunization with inactivated RSV had reduced clinical illness after live virus challenge, as measured by weight loss, illness score, and virus replication. This was associated with an augmented CD8+ cytotoxic T lymphocyte (CTL) activity, increased expression of IFN-gamma mRNA relative to IL-4 mRNA, and a higher titer of RSV-specific IgG2a in the anti-IL-4 treated mice before challenge. Anti-IL-4 administration at the time of challenge had no effects on illness, immunoglobulin isotype, or cytokine patterns. These results suggest that inhibition of IL-4 action at immunization can shift the selective activation of lymphocytes to a more Th1-like response. This cytokine milieu is associated with augmented CTL activity, which may be the factor responsible for rapid viral clearance and reduced illness at the time of remote RSV challenge.
Full text
PDF

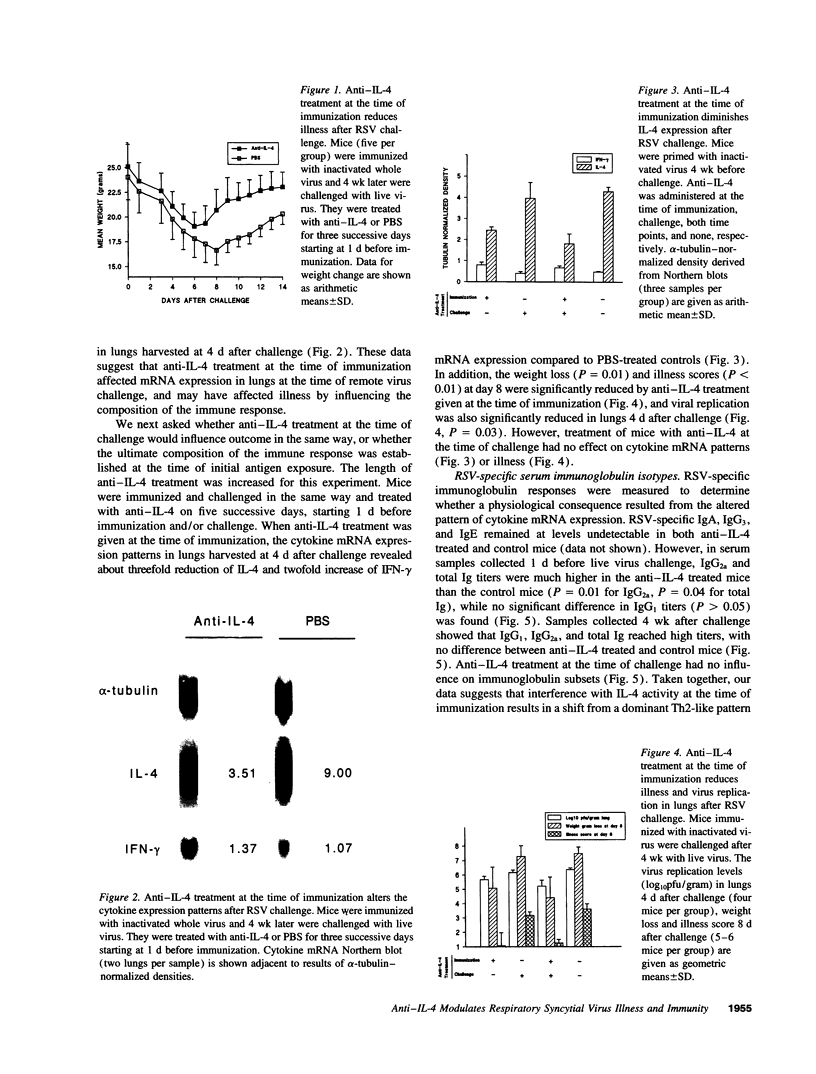
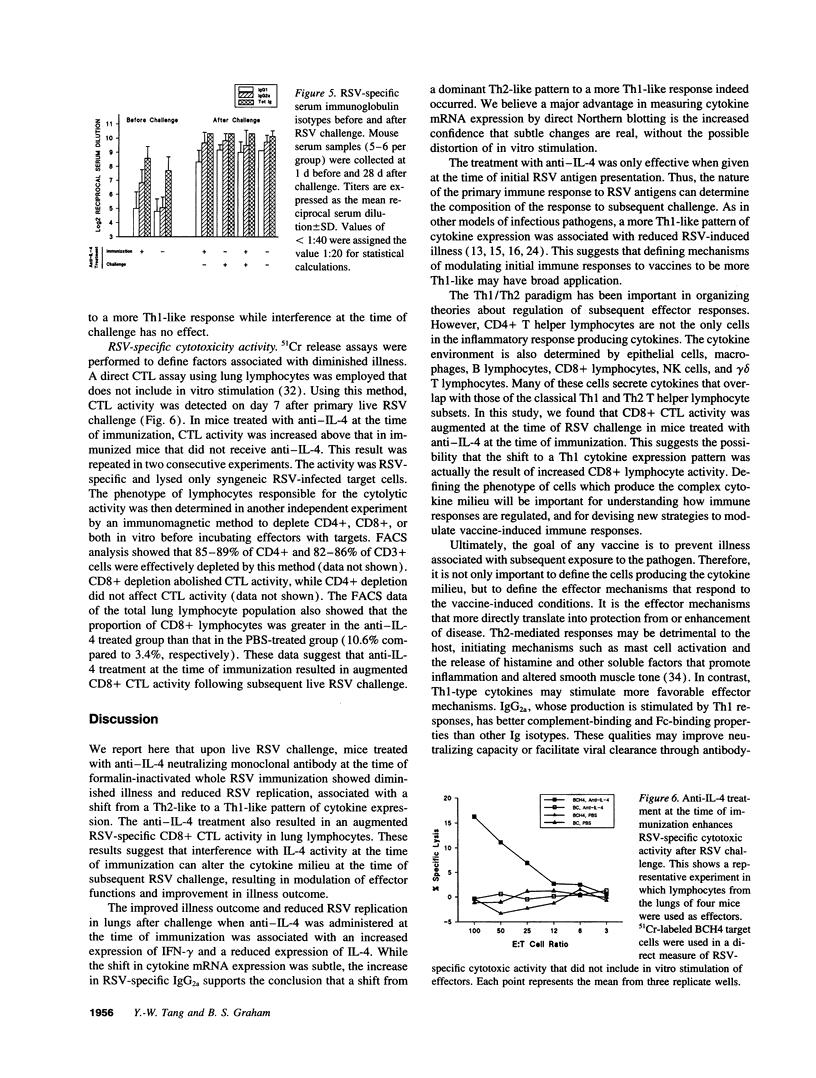


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwan W. H., Kozlowska W. J., Openshaw P. J. Distinct types of lung disease caused by functional subsets of antiviral T cells. J Exp Med. 1994 Jan 1;179(1):81–89. doi: 10.1084/jem.179.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan W. H., Record F. M., Openshaw P. J. Phenotypic and functional characterization of T cell lines specific for individual respiratory syncytial virus proteins. J Immunol. 1993 Jun 15;150(12):5211–5218. [PubMed] [Google Scholar]
- Bangham C. R., Cannon M. J., Karzon D. T., Askonas B. A. Cytotoxic T-cell response to respiratory syncytial virus in mice. J Virol. 1985 Oct;56(1):55–59. doi: 10.1128/jvi.56.1.55-59.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brun A., Gaudernack G., Sandberg S. A new method for isolation of reticulocytes: positive selection of human reticulocytes by immunomagnetic separation. Blood. 1990 Dec 1;76(11):2397–2403. [PubMed] [Google Scholar]
- Cannon M. J., Openshaw P. J., Askonas B. A. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988 Sep 1;168(3):1163–1168. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon M. J., Stott E. J., Taylor G., Askonas B. A. Clearance of persistent respiratory syncytial virus infections in immunodeficient mice following transfer of primed T cells. Immunology. 1987 Sep;62(1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Cherrie A. H., Anderson K., Wertz G. W., Openshaw P. J. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992 Apr;66(4):2102–2110. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J., Magoffin R. L., Shearer L. A., Schieble J. H., Lennette E. H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969 Apr;89(4):449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- Clerici M., Hakim F. T., Venzon D. J., Blatt S., Hendrix C. W., Wynn T. A., Shearer G. M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Invest. 1993 Mar;91(3):759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors M., Kulkarni A. B., Firestone C. Y., Holmes K. L., Morse H. C., 3rd, Sotnikov A. V., Murphy B. R. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. J Virol. 1992 Dec;66(12):7444–7451. doi: 10.1128/jvi.66.12.7444-7451.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulginiti V. A., Eller J. J., Sieber O. F., Joyner J. W., Minamitani M., Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969 Apr;89(4):435–448. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- Galli S. J. New concepts about the mast cell. N Engl J Med. 1993 Jan 28;328(4):257–265. doi: 10.1056/NEJM199301283280408. [DOI] [PubMed] [Google Scholar]
- Gazzinelli R. T., Hakim F. T., Hieny S., Shearer G. M., Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991 Jan 1;146(1):286–292. [PubMed] [Google Scholar]
- Graham B. S., Bunton L. A., Rowland J., Wright P. F., Karzon D. T. Respiratory syncytial virus infection in anti-mu-treated mice. J Virol. 1991 Sep;65(9):4936–4942. doi: 10.1128/jvi.65.9.4936-4942.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. S., Bunton L. A., Wright P. F., Karzon D. T. Reinfection of mice with respiratory syncytial virus. J Med Virol. 1991 May;34(1):7–13. doi: 10.1002/jmv.1890340103. [DOI] [PubMed] [Google Scholar]
- Graham B. S., Bunton L. A., Wright P. F., Karzon D. T. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991 Sep;88(3):1026–1033. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. S., Henderson G. S., Tang Y. W., Lu X., Neuzil K. M., Colley D. G. Priming immunization determines T helper cytokine mRNA expression patterns in lungs of mice challenged with respiratory syncytial virus. J Immunol. 1993 Aug 15;151(4):2032–2040. [PubMed] [Google Scholar]
- Griffin D. E., Ward B. J. Differential CD4 T cell activation in measles. J Infect Dis. 1993 Aug;168(2):275–281. doi: 10.1093/infdis/168.2.275. [DOI] [PubMed] [Google Scholar]
- Gross A., Ben-Sasson S. Z., Paul W. E. Anti-IL-4 diminishes in vivo priming for antigen-specific IL-4 production by T cells. J Immunol. 1993 Mar 15;150(6):2112–2120. [PubMed] [Google Scholar]
- Heinzel F. P., Sadick M. D., Holaday B. J., Coffman R. L., Locksley R. M. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med. 1989 Jan 1;169(1):59–72. doi: 10.1084/jem.169.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson G. S., Conary J. T., Davidson J. M., Stewart S. J., House F. S., McCurley T. L. A reliable method for northern blot analysis using synthetic oligonucleotide probes. Biotechniques. 1991 Feb;10(2):190–197. [PubMed] [Google Scholar]
- Isaacs D., Bangham C. R., McMichael A. J. Cell-mediated cytotoxic response to respiratory syncytial virus in infants with bronchiolitis. Lancet. 1987 Oct 3;2(8562):769–771. doi: 10.1016/s0140-6736(87)92502-5. [DOI] [PubMed] [Google Scholar]
- Kanagawa O., Vaupel B. A., Gayama S., Koehler G., Kopf M. Resistance of mice deficient in IL-4 to retrovirus-induced immunodeficiency syndrome (MAIDS) Science. 1993 Oct 8;262(5131):240–242. doi: 10.1126/science.8211142. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Mitchell R. H., Chanock R. M., Shvedoff R. A., Stewart C. E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969 Apr;89(4):405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Brandt C. D., Wright P., Hodes D., Chanock R. M., Parrott R. H. Safety and antigenicity of temperature sensitive (TS) mutant respiratory syncytial virus (RSV) in infants and children. Pediatrics. 1973 Jul;52(1):56–63. [PubMed] [Google Scholar]
- Kim H. W., Arrobio J. O., Pyles G., Brandt C. D., Camargo E., Chanock R. M., Parrott R. H. Clinical and immunological response of infants and children to administration of low-temperature adapted respiratory syncytial virus. Pediatrics. 1971 Nov;48(5):745–755. [PubMed] [Google Scholar]
- Kim H. W., Canchola J. G., Brandt C. D., Pyles G., Chanock R. M., Jensen K., Parrott R. H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969 Apr;89(4):422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kopf M., Le Gros G., Bachmann M., Lamers M. C., Bluethmann H., Köhler G. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993 Mar 18;362(6417):245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- Maziarz R. T., Mentzer S. J., Burakoff S. J., Faller D. V. Distinct effects of interferon-gamma and MHC class I surface antigen levels on resistance of the K562 tumor cell line to natural killer-mediated lysis. Cell Immunol. 1990 Oct 15;130(2):329–338. doi: 10.1016/0008-8749(90)90276-w. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Muñoz J. L., McCarthy C. A., Clark M. E., Hall C. B. Respiratory syncytial virus infection in C57BL/6 mice: clearance of virus from the lungs with virus-specific cytotoxic T cells. J Virol. 1991 Aug;65(8):4494–4497. doi: 10.1128/jvi.65.8.4494-4497.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholas J. A., Rubino K. L., Levely M. E., Adams E. G., Collins P. L. Cytolytic T-lymphocyte responses to respiratory syncytial virus: effector cell phenotype and target proteins. J Virol. 1990 Sep;64(9):4232–4241. doi: 10.1128/jvi.64.9.4232-4241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Romani L., Mocci S., Bietta C., Lanfaloni L., Puccetti P., Bistoni F. Th1 and Th2 cytokine secretion patterns in murine candidiasis: association of Th1 responses with acquired resistance. Infect Immun. 1991 Dec;59(12):4647–4654. doi: 10.1128/iai.59.12.4647-4654.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadick M. D., Heinzel F. P., Holaday B. J., Pu R. T., Dawkins R. S., Locksley R. M. Cure of murine leishmaniasis with anti-interleukin 4 monoclonal antibody. Evidence for a T cell-dependent, interferon gamma-independent mechanism. J Exp Med. 1990 Jan 1;171(1):115–127. doi: 10.1084/jem.171.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott P., Natovitz P., Coffman R. L., Pearce E., Sher A. Immunoregulation of cutaneous leishmaniasis. T cell lines that transfer protective immunity or exacerbation belong to different T helper subsets and respond to distinct parasite antigens. J Exp Med. 1988 Nov 1;168(5):1675–1684. doi: 10.1084/jem.168.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A., Coffman R. L., Hieny S., Cheever A. W. Ablation of eosinophil and IgE responses with anti-IL-5 or anti-IL-4 antibodies fails to affect immunity against Schistosoma mansoni in the mouse. J Immunol. 1990 Dec 1;145(11):3911–3916. [PubMed] [Google Scholar]
- Taylor G., Stott E. J., Hayle A. J. Cytotoxic lymphocytes in the lungs of mice infected with respiratory syncytial virus. J Gen Virol. 1985 Dec;66(Pt 12):2533–2538. doi: 10.1099/0022-1317-66-12-2533. [DOI] [PubMed] [Google Scholar]
- Wright P. F., Belshe R. B., Kim H. W., Van Voris L. P., Chanock R. M. Administration of a highly attenuated, live respiratory syncytial virus vaccine to adults and children. Infect Immun. 1982 Jul;37(1):397–400. doi: 10.1128/iai.37.1.397-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura M., Uyemura K., Deans R. J., Weinberg K., Rea T. H., Bloom B. R., Modlin R. L. Defining protective responses to pathogens: cytokine profiles in leprosy lesions. Science. 1991 Oct 11;254(5029):277–279. doi: 10.1126/science.254.5029.277. [DOI] [PubMed] [Google Scholar]




