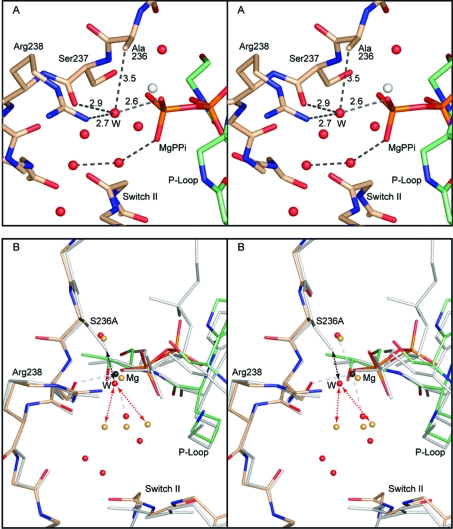
Figure 2.
Stereoviews the γ-phosphoryl binding site in the S236A·MgPPi complex and a comparison with wild-type S1dC·MgATP. Both of these complexes represent the open state of myosin. (A) shows the coordination of the water molecule that fills the cavity left by the removal of the γ-hydroxyl group of Ser236. The new water molecule is coordinated by one of the nonbridging oxygen atoms of the terminal phosphoryl group of magnesium pyrophosphate, the carbonyl oxygen of Ser237, and the guanadinium Nη of Arg238 (distances in Å). The water molecule is within van der Waals distance of the β-carbon of Ala236. (B) shows the superposition of the S236A·MgPPi complex with the wild-type S1dC·MgATP complex (PDB accession code 1FMW(41)). The S236A·MgPPi complex is depicted in color whereas the S1dC·MgATP complex is shaded in gray. This panel reveals the very close similarity between the structures of these complexes and demonstrates that the S236A mutation does not introduce a large perturbation in the overall structure of the myosin motor domain. The new water molecule in the S236A·MgPPi complex is labeled “W” for clarity. As can be seen, a water molecule could not adopt this position in the wild-type structure since it would clash with the serine hydroxyl (black dashed arrows). Introduction of the new water molecule displaces the primary waters observed in the wild-type S1dC·MgATP complex (red dashed arrows). The structures described in this figure and elsewhere were superimposed with the program uw_align (43). The coordinates for the wild-type S1dC·MgATP complex were obtained from PDB file 1FMW(41).