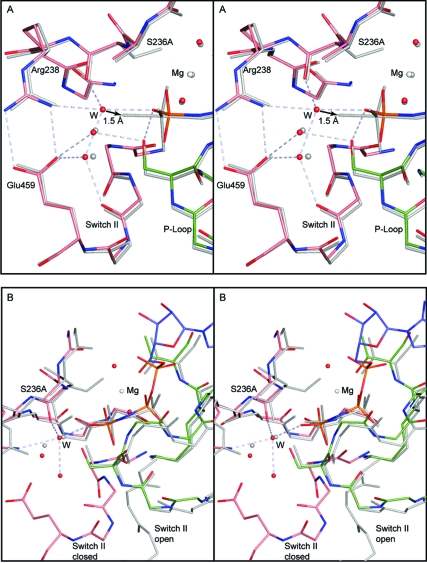
Figure 4.
Stereo comparison of (A) S236A·MgAMPPNP with S236A·MgADP·VO4 and (B) S236A·MgAMPPNP with S236A·MgPPi. The bonds in the S236A·MgAMPPNP are depicted in color, whereas the other complexes are shown in gray. In (A) the water structures for the two closed complexes of the S236A mutant are compared. These mimic the ATP and transition state for the closed conformation. The hydrogen-bonding network is shown for the water molecules in the S236A·MgAMPPNP. For clarity the hydrogen bond distances have been omitted; however, all of the distances lie within the range of 2.6−2.9 Å. The arrow indicates that the new water molecule (W) would only have to move 1.5 Å to adopt an axial position relative to the γ-phosphoryl moiety. Regardless of whether this is the nucleophile, this water molecule must move in order to form the transition state for hydrolysis. (B) shows the superposition of the open and closed states of the S236A mutant protein as represented by the MgPPi and MgAMPPNP complexes. This reveals that the new water molecule that takes the place of the serine hydroxyl adopts the same location in the open and closed complexes.