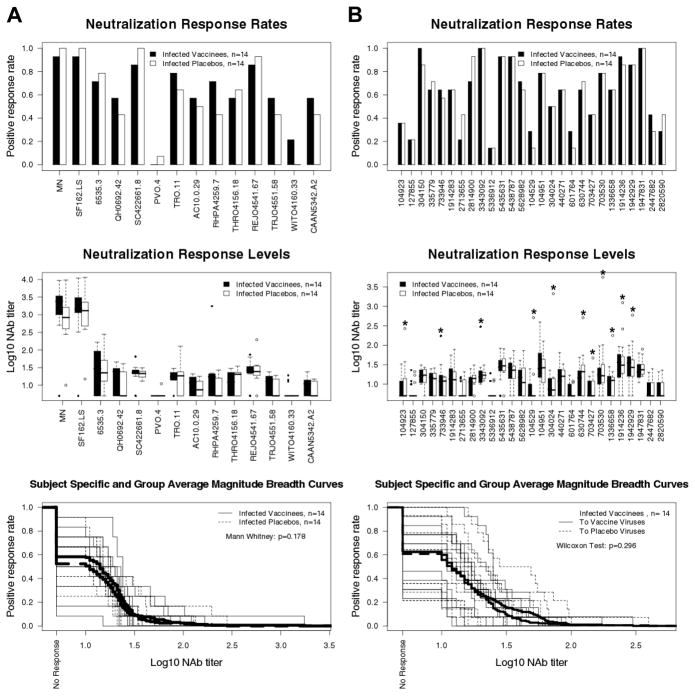
Fig. 4.
Comparison of post-infection NAb responses among vaccine and placebo recipients. NAbs were assessed in plasma samples from 14 vaccine recipients and 14 placebo recipients 12–24 months after diagnosis of infection. All subjects were antiretroviral therapy naïve at the time of plasma collection. A. Assays with MN, SF162.LS and the subtype B reference panel of tier 2 viruses. B. Assays with viruses from trial participants. In the top two diagrams, the first 13 viruses from the left are from vaccine recipients and the second 14 viruses are from placebo recipients. Autologous virus/plasma combinations in the middle diagram (Neutralization Response Levels) are indicated by an asterisk. All results in A and B were obtained in the U87.CD4.CCR5.CXCR4 assay. Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively, and are for the tier 2 viruses only.