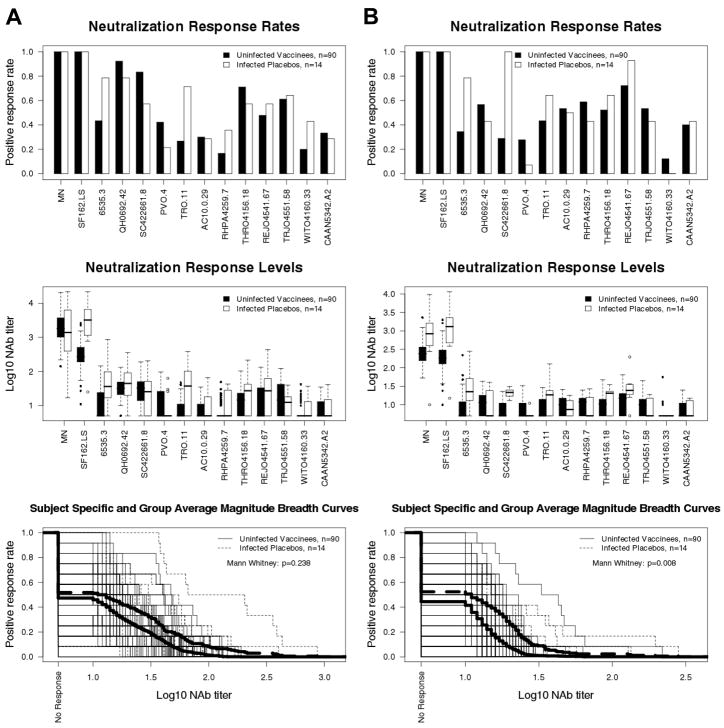
Fig. 5.
Comparison of pre-infection NAb responses in vaccine recipients to post-infection NAb responses in placebo recipients. Plasma from 90 vaccine recipients (2 weeks post fourth inoculation) and 14 placebo recipients (1–2 years post diagnosis) were assayed against MN, SF162.LS and the subtype B reference panel of tier 2 viruses. A. TZM-bl assay. B. U87.CD4.CCR5.CXCR4 assay. Subject-specific and group averages in M-B plots are shown as light and heavy lines, respectively, and are for the tier 2 viruses only.