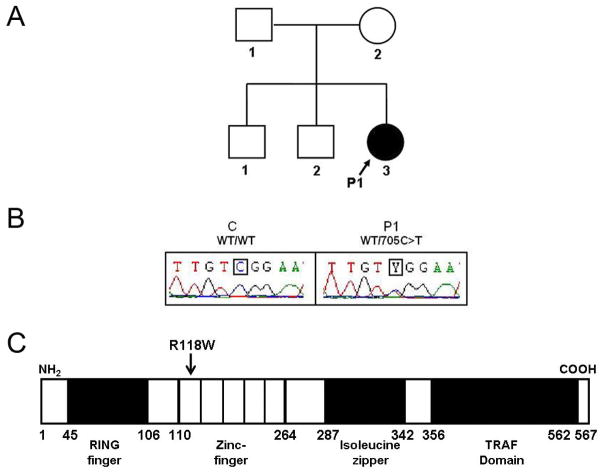
Fig. 1. Heterozygous TRAF3 mutation in a child with HSE (P1).
(A) Family pedigree, with allele segregation. The patient with HSE, in black, is heterozygous for the mutation. (B) Heterozygous c.705C>T mutation in patient 1 (P1). The sequence of the polymerase chain reaction products of genomic DNA from leukocytes of a control (C) and P1 is shown. (C) Schematic representation of TRAF3 protein structure. Human TRAF3 has eleven exons, encoding a protein composed of a ring finger and five zinc-finger domains in the N-terminal region, followed by an isoleucine zipper and a TRAF domain in the C-terminal region. P1 carries the c.705C>T mutation, which results in an arginine (R) to tryptophan (W) substitution at amino-acid position 118 (R118W) in the first zinc-finger domain.