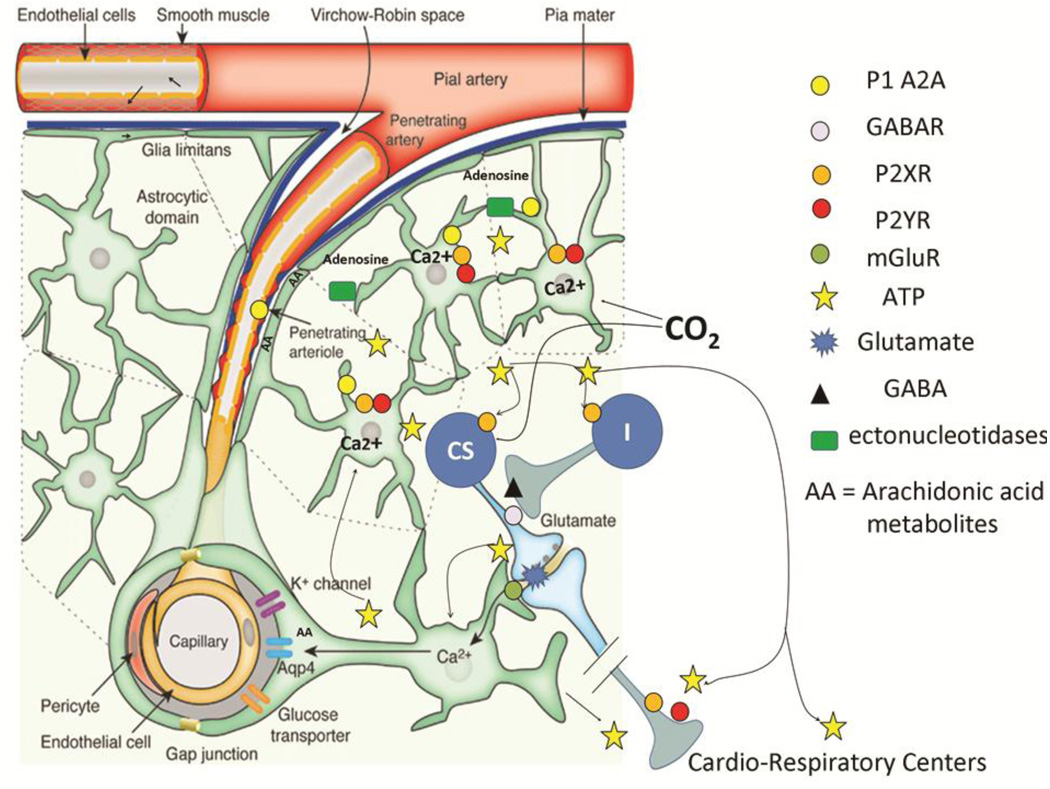
Figure 2.
A schematic drawing showing the effects of hypercapnia on ATP release and the ramification of ATP throughout the ventral medulla (adapted from (Iadecola and Nedergaard, 2007) with permission). One of the critical cellular events associated with glial ATP release is an increase in intracellular calcium that precedes vesicular fusion and release. Preliminary studies from one of our labs using the calcium indicator, calcium crimson, in GFP-expressing astrocytes suggest that intracellular calcium is increased in glia limitans in the RTN during exposure to CO2 (Erlichman, J.S.; unpublished findings). The mechanisms of ATP release appear to be varied. Recent work suggests that vesicular ATP release is via exocytotic release by lysosomes; however release may also be mediated by connexins, pannexins, or other transport mechanisms in Ca2+ independent manner (Cotrina et al., 1998; Iglesias et al., 2009; Zhang et al., 2007). Ectonucleotidases in the extracellular space generates adenosine that binds to the A2A subtype of P1 receptors, thereby augmenting dilation of pial/penetrating arteries (Langer et al., 2008; Stefan et al., 2006; Xu and Pelligrino, 2007). Purinergic activation results in the generation of vasoactive arachadonic acid metabolites (AA) (Haydon and Carmignoto, 2006; Takano et al., 2006; Zonta et al., 2003a). As the ATP/calcium cascade traverses down the vascular tree to smaller vessels, the mechanisms responsible for the dilation may rely more heavily on AA generation as opposed to the actions of adenosine.
As glial ATP migrates deeper into the neuropil, P2Y receptors on some chemosensory cells (CS) may be activated by ATP release (Mulkey et al., 2006). These cells appear to receive projections from unidentified cells that release GABA/glycine in response P2X2 receptor activation (I). ATP released in cardiovascular control centers in the ventral medulla also seems to augment the activity of these centers via P2X and P2Y receptors during hypoxia and hypercapnia.