Figure 6.
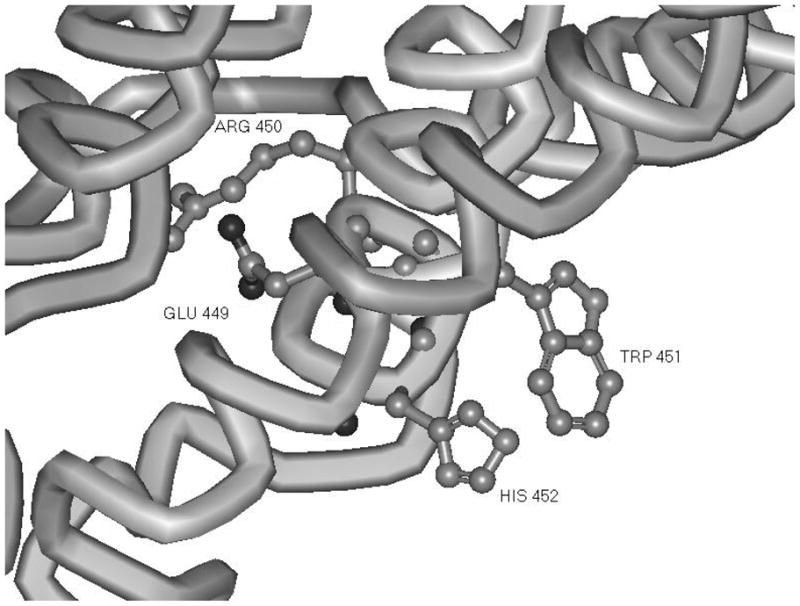
Orientation of a G-protein interaction motif (ERW)H in a model of FSHR cytoplasmic domain structure (4). Substitution of alanine at position His 452 affects neither trafficking to the membrane nor FSHR binding. This mutation results in a block to cAMP production and failure of the hormone receptor to internalize. The Glu 449 and Arg 450 are oriented into the center of the transmembrane domain helices whereas side chains of both His 452 and Trp 451 are oriented towards solvent.
