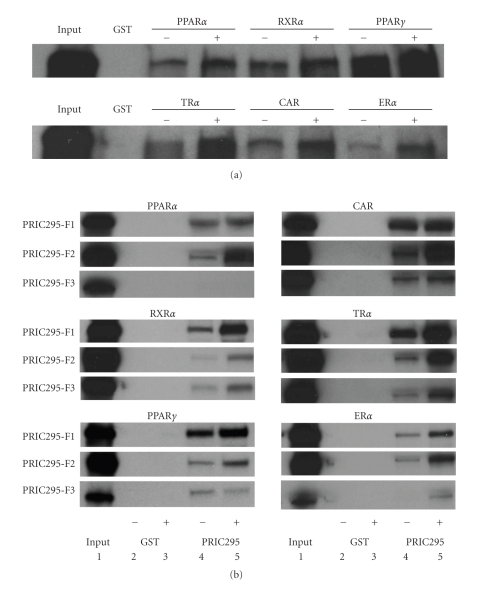
Figure 4.
PRIC295 interactions with nuclear receptors. (a) In vitro interaction of radiolabeled full-length PRIC295 with nuclear receptors PPARα, RXRα, PPARγ, TRα, CAR, and ERα. PRIC295 was radiolabeled using 35S-methionine (Perkin Elmer) and incubated with each shown receptor fusion protein in GST-pulldown assay. (−) minus indicates in vitro binding interaction in the absence of cognate ligand and (+) plus indicates the presence of receptor-specific ligand during the in vitro binding interaction. Ligands used: Wy-14,643 for PPARα; 9-cis-retinoic acid for RXRα; rosiglitazone for PPARγ; triiodothyronine (T3)TRα; 1,4-Bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP) for CAR; 17-β-estradiol for ERα. All ligands were used at a concentration of 100 μM. (b) Interaction of radiolabeled PRIC295 fragments ΔPRIC2951–915 (F1), ΔPRIC295840–1815 (F2), and ΔPRIC2951740–2671 (F3) with GST-fusion nuclear receptor proteins. Loading for each lane is indicated at the base of each panel. Lane 1 input; lane 2 GST alone without ligand; lane 3 GST alone with receptor-specific ligand; lane 4 GST-receptor fusion in the absence ligand; lane 5 in the presence of receptor-specific ligand. All ligands used are as previously described in (a).