Figure 1.
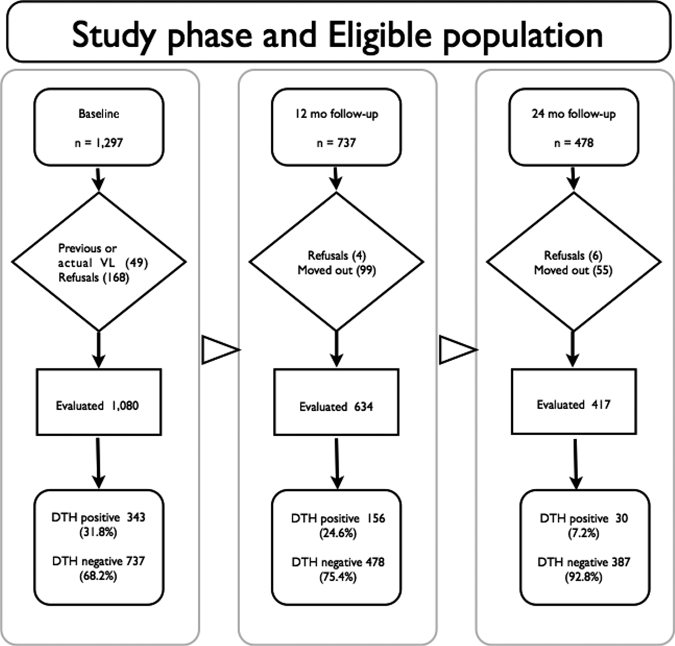
Study flow chart. The study was divided into three phases, baseline, 12-month follow-up, and 24-month follow-up with one cohort from the endemic area of visceral leishmaniasis (VL) in Maranhão State, Brazil to address anti-Leishmania delayed-type hypersensitivity (DTH) and antibody responsiveness to Lutzomyia longipalpis salivary proteins. At the end of the first phase there were 737 DTH-negative children who constituted the eligible population for the phase 2 (12-month follow-up). The 478 children who were DTH at phase 2 included the eligible population of phase 3 (24-month follow-up). The study design details are described in the text.
