Abstract
Evidence for the neurotoxicity of extended exposure to low levels of aluminum salts is described using an animal model treated with aluminum at low levels reflecting those found in found in some water supplies. Emphasis is given to the potential role of aluminum in acceleration and promotion of some indices characteristic of brain aging. These hallmarks include the appearance of excess levels of inflammation in specific brain areas. Aluminum salts can increase levels of glial activation, inflammatory cytokines and amyloid precursor protein within the brain. Both normal brain aging and to a greater extent, Alzheimer’s disease are associated with elevated basal levels of markers for inflammation. These are not attributable to obvious exogenous stimuli and may reflect the lifespan history of the organism’s immune responses. It is possible that aluminum salts can act as a subtle promoter of such apparently unprovoked responses.
Keywords: Aluminum, drinking water brain aging, Alzheimer’s disease
Introduction
This review on the potential neurotoxic hazard posed by aluminum focuses on distinguishing between those aspects of aluminum neurotoxicity that are widely accepted and not controversial, and those aspects that are suggestive but not fully established. The work of this laboratory over the past 12 years is described and this falls into the latter classification, adding to the body of evidence increasingly implicating aluminum as a potentially hazardous environmental agent.
The most accepted facts relating to aluminum include: i) It’s widespread prevalence and level of human consumption, ii) The known neurotoxicity of high levels of aluminum, and iii) A repeated epidemiological correlation between ingested aluminum and the incidence of Alzheimer’s disease. Another relevant area of widespread consensus is the tendency of the aging brain to express elevated levels of inflammation and the further exacerbation of this state in several neurodegenerative diseases.
Less accepted is the evidence that aluminum can be a causal factor in promoting Alzheimer’s disease. This area has been handicapped by earlier erroneous reports of heightened levels of aluminum in amyloid plaques. Tissue aluminum levels are notoriously difficult to determine and later more reliable findings leading to similar conclusions have been overshadowed by these original reports. The main objective of our more recent studies has been to study the effects of extended exposure of experimental animals to levels of aluminum that have relevance for the human population. Demonstration that these can provoke cerebral inflammatory responses resembling those that are found with brain senescence, can provide a mechanistic link to epidemiological reports.
1. Aluminum is environmentally prevalent and ingested by humans
Aluminum (Al) salts are used as a coagulant for purification of drinking water and as a food additive. The most common form of human exposure to Al3+ is absorption through the gastrointestinal tract. The rate of absorption is approximately 0.22% (Priest et al, 1998) and once in the blood, approximately 90% of the metal is bound to transferrin (Cabezuelo et al., 1997; Harris et al., 2003). Al3+ can pass the blood-brain barrier by receptor-mediated endocytosis of the Fe-carrier protein and in rats approximately 0.005% of the metal complexes enter the brain (Yokel et al., 2001).
Until recently aluminum in the environment was considered harmless, because in solution, Al3+ salts form monomeric hydroxy compounds which start to form polymeric and colloidal particles as the solution ages. Because of the formation of these insoluble aluminum species, it was assumed that absorption would be limited and thus the metal would be innocuous. However, aluminum compounds have been shown to be toxic to animals (Sparling and Campbell, 1997) and there has been a rising concern over the metal’s potential adverse health effects (Lazarte et al., 1997). The increasing prevalence of acid rain can lead to the release of greater amounts of aluminum salts from insoluble minerals, leading to greater bioavailability (Smith, 1996).
2. There is an epidemiological relation between chronic aluminum exposure and the incidence of Alzheimer’s disease
The presence of excess aluminum in the brains of patients with Alzheimer’s disease has been described. Earlier reports (Perl et al., 1982) have been confirmed using more sophisticated analytical procedures (Bouras et al., 1997, Andrasi et al., 2005). Elevated aluminum levels have also been reported in other less common neurological disorders such as the Guamanian Parkinsonian-ALS constellation and Hallervorden-Spatz disease (Eidelberg et al., 1987, Garruto et al., 1989). This has raised the question as to whether the metal may play a role in several neurological disorders (Kawahara, 2005). This issue is unresolved since several conflicting reports exist (Xu et al., 1992). However, chelation therapy in order to reduce the aluminum burden in Alzheimer patients, has been reported as beneficial (McLachlan et al., 1991). In view of the many adverse effects of deferoxamine, new Al-specific chelators for potential use in AD treatment, have recently been developed (Shin et al., 2003)
An increasing number of epidemiological reports relate the aluminum content of drinking water with increasing incidence of neurological disease. A study by McLachlan et al. (1996) correlated the risk of developing Alzheimer’s disease with residing in areas where aluminum concentrations in the municipal drinking water are 100 μg/L or greater. A dose-response correlation between an increasing concentration of Al in the drinking water and a higher risk of developing Alzheimer’s disease (AD) was found. Another study, looking at elderly populations exposed to Al3+ in drinking water (100 μg/L), also reported a similar link between exposure and the prevalence of AD (Rondeau et al., 2000). A comprehensive literature survey has found thirteen reports concerning a significant association between residing in areas where aluminum concentrations in the municipal drinking water are high and an increase in the incidence of AD, and a meta-analysis, integrating results from many sources, reveals that this association is significant (Flaten, 2001). Meta-analysis has limitations as a methodology but these findings have been confirmed in a recently published study involving a 15-year follow up of a large population (Rondeau et al., 2009).
Correlative changes are never sufficient to imply causation. Proposals have been made that aluminum entry into the brain is a secondary epiphenomenon, consequent to damage to the blood brain barrier. However, dialysis encephalopathy can be treated with deferoxamine with good results suggesting that Al is directly neurotoxic (Abou-Donia, 1992). Treatment of aluminum-related bone disease with deferoxamine which mobilizes bone-Al and elevates serum Al3+, can precipitate dementia (Sherrard et al., 1988). While this chelator is not specific for aluminum, in both these instances, a causal relation between circulating Al3+ and dementia is suggested.
We have found that some biological effects of colloidal aluminum may resemble that of the toxic 25-35 β-amyloid fragment (Yang et al., 1999). Interactions between aluminum and transition metals may parallel those between amyloid and transition metals, such as the ability of copper salts to effect aggregation of β-amyloid (Atwood et al., 1998). Aluminum suspensions like other colloids can attract transition metals to their surfaces and this can promote valence flux leading to production of reactive oxygen species (Bondy et al., 1998). Aluminum complexes can also potentiate the rate of aggregation of β-amyloid and enhance the toxicity of this peptide (Exley, 1997, Bondy and Truong, 1999). Finally, dietary exposure to Al can exacerbate Aβ deposition and plaque formation in the brain of transgenic mice over-expressing amyloid precursor protein (APP) (Pratico et al., 2002), from which Aβ is initially generated. Alzheimer’s disease is characterized by brain depositions of the toxic amyloid β-peptide (Aβ) to form amyloid plaques. In the brain of AD patients, reactive microglia, producing proinflammatory cytokines and acute phase proteins, are associated with Aβ-containing neuritic plaques (Mrak et al., 1995; Styren et al., 1998).
There has been difficulty in development of a rationale concerning mechanisms underlying aluminum neurotoxicity. Various suggestions have been made, including the possibility that insoluble aluminum complexes may induce glial activation and macrophage activity (Evans et al., 1992, Garrel et al., 1994, Shigematsu and McGeer, 1992). This is supported by the observation that, in rats intracerebroventricularly injected with Al3+, complexes of the metal accumulate largely in the striatum, and this is accompanied by gliosis (Platt et al., 2001). Upon postmortem analysis of a chronic renal failure patient using phosphate-binding Al-hydroxy gels for a prolonged period, increased proliferation of microglia and astrocytes in the same region of the brain was also found (Shirabe et al., 2002). This patient developed Al-induced encephalopathy nine months prior to death. Prior work from our laboratory has found that aluminum increases cell proliferation, cytokine secretion, and NF-κB activation in human glioblastoma cells (Campbell et al., 2002). Levels of activated NF-κB, accompanied by a significant increase in inflammatory cytokines, are also increased in the brains of mice following consumption of Al lactate in drinking water (Campbell et al., 2004, Becaria et al., 2006). It is noteworthy that, when TNF-α is elevated in many organs by a systemic inflammatory stimulus, it remains elevated in brain much longer than in other tissues (Qin et al., 2007).
3. Aluminum exposure may also promote the onset of Parkinson’s disease
There is evidence relating aluminum exposure to Parkinson’s disease. An epidemiological study has found a correlation between this disorder and Al exposure (Altschuler, 1999). Al concentrations are elevated in dopamine-related brain regions of PD patients (Yasui et al.,1992) and occupational exposure to aluminum may constitute a risk factor for PD (Gorell et al.,1999). In a case of encephalopathy due to treatment with Al-hydroxy gels in renal failure, postmortem analysis revealed gliosis to be especially pronounced in striatal regions (Shirabe et al., 2002). PD is a disorder increasingly recognized as involving inflammatory events (Selley et al., 2005), microglial activation and increased levels of pro-inflammatory cytokines (Nagatsu and Sawada, 2005). Non-steroidal anti-inflammatory drugs (NSAIDs) have been shown to reduce the risk of developing PD (Hald and Lotharius, 2005).
There is some experimental evidence that Al exposure can adversely affect the dopaminergic system. Extended exposure to 100μM Al lactate increased striatal levels of the dopamine metabolite, DOPAC (Li et al., 2008). This was accompanied by a non-significant trend toward depressed levels of dopamine, suggesting that exposure to Al may cause increased turnover of dopamine. This led to testing the effect of challenge of Al-treated mice with MPTP, a dopaminergic neurotoxin. While neither Al nor MPTP treatment altered cortical activation levels of NF-κB, the two stressors in combination significantly elevated levels of this transcription factor. The p50 subunit of NF-κB was also higher in animals treated with both toxicants. Similarly, treatment of mice with both agents together but not to either alone, led to pronounced astroglial activation in the circumventricular region (Fig. 1). Thus, aluminum and MPTP interact synergistically in enhancing dopamine turnover, levels of GFAP and activation of NF-| B (Li et al., 2008). There are reports of potentiation of MPTP effects by other neurotoxicants including 3-niroproprionic acid (Ahuja et al., 2008). It should be noted that astroglial activation can occur in a time and dose dependant manner using MPTP alone (Ho and Blum, 1998). The rise of GFAP seen after MPTP treatment may be more persistent in female mice (Ciesielska et al., 2009, Ookubo et al., 2008), while all the mice used in our study were male. Also, it is important to recognize that low level inflammatory responses including microglial activation can have beneficial effects in the brain (Kriz, 2006).
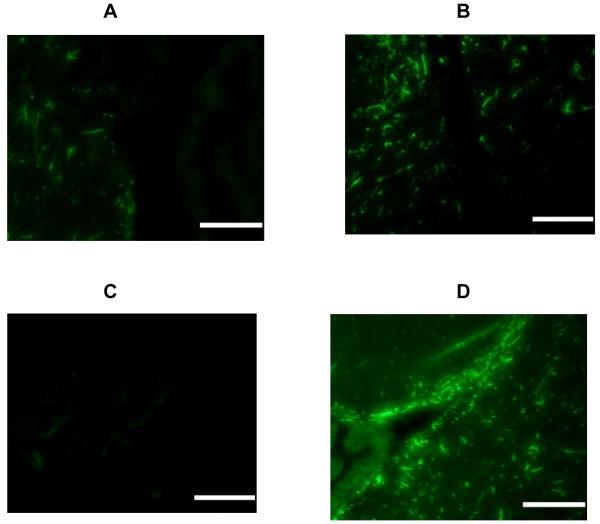
Fig. 1.
Immunofluorescent staining for GFAP in the periventricular area. Sections derived from: control animals (A), Al exposed mice (B), mice treated with MPTP (C) and mice dosed with both Al and MPTP (D). Bars represent 100 microns. Each section derived from a representative mouse from a group of 3 receiving identical treatment.
A further study of potential interactions of Al and MPTP was performed. Treatment with both toxicants together resulted in an increase in the level of AP-1 binding to DNA and elevation of the content of activated, phosphorylated JNK-1 (P-JNK) (Li et al., 2008). A similar increase was seen with phosphorylated p38 in animals treated with both Al lactate and MPTP. Such evidence of mobilization of a specific transcription factor following activation of a kinase signaling pathway was only found when both agents were present together. This implies that aluminum and MPTP can act synergistically. The utility of an acute challenge when considering the consequences of extended exposures to low levels of an environmentally prevalent xenobiotic agent, is evidenced by this study. The findings suggest dopaminergic neuronal circuitry is vulnerable to low levels of aluminum. This may be due to the relatively high glial content in striatal areas and the globus pallidus. It may also be related to the sensitivity of dopamine neurons to oxidative damage.
4. Acute exposure to aluminum can cause clinical neurotoxicity
Aluminum (Al) is the third most abundant element in the earth’s crust but is not an essential trace metal for mammals. However the concentrations in the body are sufficient to modify the activity levels of several key enzymes and second messenger pathways. Plasma concentrations of Al3+, as high as 0.4 μM, have been reported in humans (Kausz et al., 1999) and orally ingested aluminum salts have been shown to lead to the deposition of Al compounds in the brain (Bowdler et al., 1979). Aluminum levels in brain increase with age (Jansson, 2001). The possibility of Al3+ being a causative agent in neurodegenerative diseases was originally raised by several findings suggesting that the metal is not innocuous in a physiological milieu. The occurrence of aluminum-induced dialysis encephalopathy in man following hemodialysis, is accompanied by elevated levels of aluminum in the brain (Russo et al., 1992) and recovery is facilitated by application of an Al chelator (Erasmus et al., 1995). Aluminum-induced encephalopathy has also been found in renal failure patients, who have undergone bladder irrigation with 1% alum (Phelps et al., 1999). These findings suggest that prolonged exposure to the metal can have adverse consequences to human health. The development of an encephalopathy, characterized by cognitive deficits, in-coordination, tremor and spinocerebellar degeneration, among workers in the aluminum industry (Polizzi et al., 2002) also indicates that exposure to the metal can be profoundly deleterious. Abnormal neurological symptoms have been observed in several patients receiving intramuscular injections of Al-containing vaccines and the WHO Vaccine Safety Advisory Committee has recognized that there may be a subset of predisposed individuals who may be sensitive to Al-containing adjuvant (Authier et al., 2001). When mice were injected with adjuvants containing aluminum in amounts equivalent to those given to US military service personnel, neuroinflammation and cell loss were found in spinal cord and motor cortex, together with memorial deficits were found (Petrik et al., 2007). Other sporadic cases of aluminum poisoning include a seizure disorder of accompanied by progressive cognitive decline, ataxia, and dysarthria consequent to the ‘cooking’ of a methadone solution in an aluminum pot to reduce the volume followed by intravenous injection. This led to very elevated levels of serum aluminum (Friesen et al., 2006).
During a mishap in Camelford, England, Al levels in drinking water supplies were several millimolar for several weeks and subsequent behavioral testing of some of the affected population revealing significant cognitive deficits (Altmann et al., 1999). These clinical findings are paralleled in an animal model where systemically administered aluminum caused behavioral deficits including in-coordination (Bowdler et al., 1979) and cognitive and morphological changes in the CNS of treated animals (Miu et al., 2004).
5. Elevated levels of intrinsic inflammation are associated with neural aging and this is exacerbated in several neurodegenerative diseases
Senescence of the brain is associated with increased levels of factors reflecting inflammation (David et al., 1997; Streit et al., 1999, Sharman et al., 2002; Sharman and Bondy, 2004, Bondy and Sharman, 2007). In age-related neurodegenerative disorders, such as AD and PD, enhancement of inflammatory processes is thought to significantly contribute to pathogenic events. The number of activated astrocytes is increased in AD and these are associated with senile plaques and with cerebral microvessels (Cullen et al. 1997). In the hippocampus of AD patients, there is an up- regulation of proinflammatory genes (Colangelo et al., 2002), and levels of cytokines are elevated in the brain (Zhao et al., 2003) as well as cerebrospinal fluid and plasma (Sun et al., 2003) of AD patients. Such age-related increased neuroinflammation has adverse consequences in that the brain is sensitized to the effects of infection or stress (Sparkman and Johnson, 2008).
A very large proportion of the genes whose expression are significantly increased with age, are related to immune function (Sharman et al., 2004, 2007, Perreau et al., 2007). Thus, basal levels of mRNA for IL-6 and TNF-α are over 10 fold higher in 26 month old than in 5 month-old B6C3F1 mice (Sharman et al., 2002), and protein levels are correspondingly elevated. However, heightened basal immune activity in the brain of the aged animal is not accompanied by an elevated response to an inflammatory stimulus of exogenous origin. In fact, the reactivity of the immune system to such a material as lipopolysaccharide is considerably decreased in the elderly mouse (Sharman et al., 2002). It is likely that attenuation of the signal/noise ratio of immune defenses with age, implies an impaired ability to mount an effective response to pathogens. Chronically sustained low-level immune responsivity is likely to produce adverse effects. The harmfulness of an extended and ineffective immune response is well illustrated in the case of lung where the continuing presence of mineral particles such as silica leads to a futile phagocytic attack by alveolar macrophages on such irresolvable foci, which ultimately leads to severe pathological changes involving inflammatory cytokines (Hamilton et al., 2008).
6. Low levels of aluminum in drinking water of experimental animals, elevate basal levels of inflammatory activity within the CNS
The above findings with the human glioblastoma cells, as well as the epidemiological studies linking an increase in the risk of developing AD in regions where the concentrations of Al3+ in the drinking water are high, formed the rationale for conducting experiments involving extended exposure of animals to relevant levels of an aluminum salt in the drinking water. The selection of levels of Al was based on reported levels of Al in drinking water which can be as high as 10 μM in some municipal waters with excursions up to 25-230 μM recorded in Ontario and Texas (Nieboer et al., 1995, Forbes and Hill, 1998, Cech and Montrera, 2000).
Adult mice were exposed for ten weeks to low levels of aluminum lactate in their drinking water and indices of an inflammatory response in the brain were sought. All levels of aluminum used did not affect body weight. Concentrations of cytokines and their mRNAs were quantitated in cerebral homogenates and immunohistochemical techniques were used to allow a more precise localization of changes occurring. Even at the lower levels of aluminum used, inflammatory responses were apparent (Campbell et al., 2004).
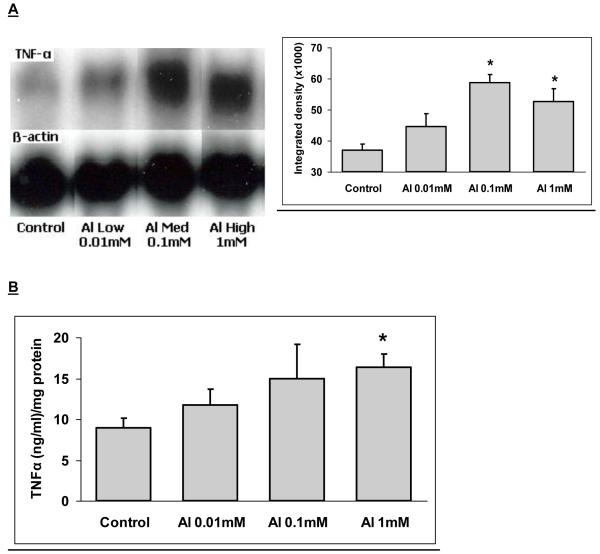
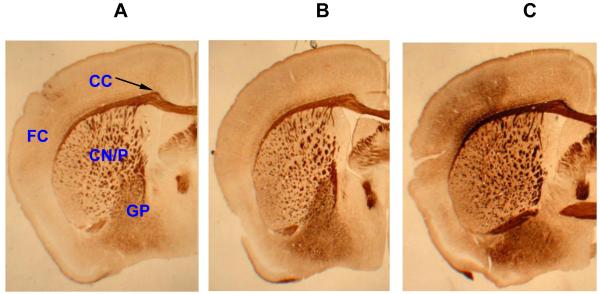
Levels of the inflammatory cytokines, TNF-α and IL-1α were elevated in response to Al lactate in a dose-dependant manner and for TNF-α this was associated with greater expression of the corresponding mRNA, (Fig. 2). Levels of amyloid precursor protein (APP) were also significantly elevated (Fig. 3). Immunohistochemical evidence of enhanced astrocyte activation proportional to aluminum dose, was found in the brains of the treated animals (Campbell et al., 2004). A parallel result was found using a stain for microglial activation (Fig. 4). All of these findings support the concept of the active role for glia in the cerebral response to aluminum. Furthermore, since TNF-α and IL-1α levels in the liver or serum of treated animals were unchanged, these changes appear to reflect a specific effect on the brain rather than a generalized inflammatory response.
Fig. 2.
A. Northern blot of TNF-α in the brain of Al-exposed mice. β-actin was used to insure equal loading of all samples. Integrated density of the blots. β-actin intensity was used to adjust the values. B. Levels of TNF-α in the cytoplasmic fraction of the brain after treatment with Al. Bars represent mean of 6 individual determinations ± SE. *: Value is significantly different (p < 0.05) from the control.
Fig. 3.
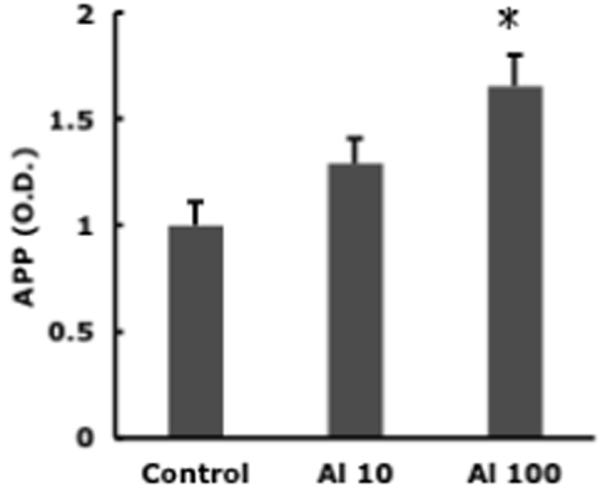
Cortical APP concentration in mice receiving 0, 10, or 100 mM Al lactate in drinking water. Bars represent mean of 6 individual determinations ± SE. * = different (p < 0.05) from the control.
Fig. 4.
Microglial activation in the brain of Al lactate-exposed animals. (A) Control, (B) Al lactate (0.01 mM), (C) Al (0.1 mM), FC = Frontal cortex, CC = corpus callosum, CN/P = caudate nucleus/putamen, GP = globus pallidus. 20x magnification.
Both lipid peroxidation and nNOS in cortex were elevated (Becaria et al., 2006). These may be considered to reflect pro-oxidant changes. The effect of Al on lipid peroxidation was especially pronounced, and significant even in the group receiving the lowest content of Al in water (10μM Al). nNOS is primarily an index of neuronal oxidant events, while iNOS (to be used in future) may primarily reflect glial activation.
There is also a recent report of the functional impairment of spatial memory discrimination in rats following to prolonged exposure to dietary aluminum corresponding to the urban American dietary aluminum range (Walton, 2009). Thus the inflammatory changes that we have found could result in cognitive deficits.
Summary
While the potential of aluminum for promoting neurodegenerative disease remains controversial, the following statements, which form the headings of the some of the above discussion, are indisputable.
Aluminum is environmentally prevalent and ingested by humans.
Acute exposure to aluminum can cause clinical neurotoxicity.
Elevated levels of intrinsic inflammation are associated with neural aging and this is exacerbated in several neurodegenerative diseases.
Low levels of aluminum in drinking water of experimental animals, elevate basal levels of inflammatory activity within the CNS.
The median age of the United States population is rapidly increasing and there is expectation of a corresponding increase in the incidence of Alzheimer’s disease, Parkinson’s disease and other chronic neurodegenerative diseases. Aging forms an essential platform for the development of such disorders and acceleration of the changes found with normal brain aging could promote their clinical onset. One of the most rewarding approaches to mitigation of the societal effects of these age-associated neurological diseases lies in the identification and mitigation of extrinsic factors which accelerate changes associated with normal cerebral senescence that are not specifically associated with any neurological disease. Aluminum is a strong candidate for consideration as a subtle promoter of events typically associated with brain aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou-Donia MB. Metals. In: Abou-Donia MB, editor. Neurotoxicology. CRC Press; Boca Raton FL: 1992. pp. 363–93. [Google Scholar]
- Ahuja M, Bishnoi M, Chopra K. Protective effect of minocycline, a semi-synthetic second-generation tetracycline against 3-nitropropionic acid (3-NP)-induced neurotoxicity. Toxicology. 2008;244:111–22. doi: 10.1016/j.tox.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Altmann P, Cunningham J, Dhanesha U, Ballard M, Thompson J, Marsh F. Disturbance of cerebral function in people exposed to drinking water contaminated with aluminium sulphate: retrospective study of the Camelford water. Brit Med J. 1999;319:807–11. doi: 10.1136/bmj.319.7213.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasi E, Pali N, Molnar Z, Kosel S. Brain aluminum, magnesium and phosphorus contents of control and Alzheimer-diseased patients. J Alzheimers Dis. 2005;7:273–84. doi: 10.3233/jad-2005-7402. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer abeta by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–26. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- Authier FJ, Cherin P, Creange A, Bonnotte B, Ferrer X, Abdelmoumni D, Ranoux D, Pelletier J, Figarella-Branger D, Granel B, Maisonobe T, Coquet M, Degos JD, Gherardi RK. Central nervous system disease in patients with macrophagic myofasciitis. Brain. 2001;124:974–83. doi: 10.1093/brain/124.5.974. [DOI] [PubMed] [Google Scholar]
- Becaria A, Lahiri DK, Bondy SC, Chen DM, Hamadeh A, Li H, Taylor R, Campbell A. Aluminum and copper in drinking water enhance chronic inflammatory of oxidative events specifically in the brain. J Neururoimmunol. 2006;176:16–23. doi: 10.1016/j.jneuroim.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Guo-Ross SX, Pien J. Mechanisms underlying aluminum-induced potentiation of oxidant properties of transition metals. Neurotoxicology. 1998;19:65–72. [PubMed] [Google Scholar]
- Bondy SC, Truong A. Potentiation of beta-folding of β-amyloid peptide 25-35 by aluminum salts. Neurosci Lett. 1999;267:25–8. doi: 10.1016/s0304-3940(99)00307-9. [DOI] [PubMed] [Google Scholar]
- Bondy SC, Sharman EH. Melatonin and the aging brain. Neurochem Int. 2007;50:571–80. doi: 10.1016/j.neuint.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Bouras C, Giannakopoulos P, Good PF, Hsu A, Hof PR, Perl DP. A laser microprobe mass analysis of brain aluminum and iron in dementia pugilistica: comparison with Alzheimer’s disease. Eur Neurol. 1997;38:53–8. doi: 10.1159/000112903. [DOI] [PubMed] [Google Scholar]
- Bowdler NC, Beasley DS, Fritze EC, Goulette AM, Hatton JD, Hessian J, Ostman DL, Rugg DJ, Schmittman CJ. Behavioural effects of aluminum ingestion on animal and human subjects. Pharmacol Biochem Behav. 1979;10:505–12. doi: 10.1016/0091-3057(79)90225-9. [DOI] [PubMed] [Google Scholar]
- Campbell A, Yang Y, Tsai-Turton M, Bondy SC. Aluminum induces reactive oxygen species formation and NF-κB activation in human glioblastoma cells. Brain Res. 2002;933:60–2. doi: 10.1016/s0006-8993(02)02305-3. [DOI] [PubMed] [Google Scholar]
- Campbell A, Becaria A, Sharman K, Bondy SC. Chronic exposure to aluminum in drinking water increases inflammatory parameters selectively in the brain. J Neurosci Res. 2004;75:565–72. doi: 10.1002/jnr.10877. [DOI] [PubMed] [Google Scholar]
- Cech I, Montera J. Spatial variations in total aluminum concentrations in drinking water supplies studied by geographic information system (GIS) methods. Water Res. 2000;34:270–312. [Google Scholar]
- Ciesielska A, Joniec I, Kurkowska-Jastrzebska I, Cudna A, Przybyłkowski A, Członkowska A, Członkowski A. The impact of age and gender on the striatal astrocytes activation in murine model of Parkinson’s disease. Inflamm Res. 2009;58:747–53. doi: 10.1007/s00011-009-0026-6. [DOI] [PubMed] [Google Scholar]
- Colangelo V, Schurr J, Ball MJ, Pelaez RP, Bazan NG, Lukiw WJ. Gene Expression profiling of 12633 genes in Alzheimer hippocampal CA1: transcription and neurotrophic factor down-regulation and up-regulation of apoptotic and pro-inflammatory signaling. J Neurosci Res. 2002;70:462–73. doi: 10.1002/jnr.10351. [DOI] [PubMed] [Google Scholar]
- Cullen KM. Perivascular astrocytes within Alzheimer’s disease plaques. Neuroreport. 1997;8:1961–6. doi: 10.1097/00001756-199705260-00033. [DOI] [PubMed] [Google Scholar]
- David JP, Ghozali F, Fallet-Bianco C, Wattez A, Delaire S, Boniface B, Di Menza C, Delacourte A. Glial reaction in the hippocampal formation is highly concentrated with aging in human brain. Neurosci Lett. 1997;235:53–6. doi: 10.1016/s0304-3940(97)00708-8. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Sotrel A, Joachim C, Selkoe D, Forman A, Pendlebury WW, Perl DP. Adult onset Hallervorden-Spatz disease with neurofibrillary pathology. Brain. 1987;110:993–1013. doi: 10.1093/brain/110.4.993. [DOI] [PubMed] [Google Scholar]
- Erasmus RT, Kusnir J, Stevenson WC, Lobo P, Herman MM, Wills MR, Savory J. Hyperaluminemia associated with liver transplantation and acute renal failure. Clin Transplant. 1995;9:307–11. [PubMed] [Google Scholar]
- Evans PH, Peterhans E, Burge T, Klinowski J. Aluminosilicate-induced free radical generation by murine brain glial cells in vitro: potential significance in the aetiopathogenesis of Alzheimer’s dementia. Dementia. 1992;3:1–6. [Google Scholar]
- Exley C. ATP-promoted amyloidosis of an amyloid β-peptide. Neuroreport. 1997;8:3411–4. doi: 10.1097/00001756-199710200-00043. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Diguet E, Bioulac B, Tison F. MPTP potentiates 3-nitropropionic acid-induced striatal damage in mice: reference to striatonigral degeneration. Exp Neurol. 2004;185:47–62. doi: 10.1016/j.expneurol.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Flaten TP. Aluminium as a risk factor in Alzheimer’s disease, with emphasis on drinking water. Brain Res Bull. 2001;55:187–196. doi: 10.1016/s0361-9230(01)00459-2. [DOI] [PubMed] [Google Scholar]
- Forbes WF, Hill GB. Is exposure to aluminum a risk factor for the development of Alzheimer disease?--Yes. Arch Neurol. 1998;55:740–1. doi: 10.1001/archneur.55.5.740. [DOI] [PubMed] [Google Scholar]
- Friesen MS, Purssell RA, Gair RD. Aluminum toxicity following IV use of oral methadone solution. Clin Toxicol. 2006;44:307–14. doi: 10.1080/15563650600637077. [DOI] [PubMed] [Google Scholar]
- Garrel C, Lafond JL, Guiraud P, Faure P, Favier A. Induction of production of nitric oxide in microglial cells by insoluble form of aluminum. Ann New York Acad Sci. 1994;738:455–61. doi: 10.1111/j.1749-6632.1994.tb21837.x. [DOI] [PubMed] [Google Scholar]
- Garruto RM, Shankar SK, Yanagihara R, Salazar AM, Amyx HL, Gajdusek DC. Low-calcium, high-aluminum diet-induced motor neuron pathology in cynomolgus monkeys. Acta Neuropathol. (Berl) 1988;78:210–9. doi: 10.1007/BF00688211. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Rybicki BA, Cole Johnson C, Peterson EL. Occupational exposure to specific metals (manganese, copper, lead, iron, mercury, zinc, aluminum and others) appears to be a risk factor for Parkinson’s disease (PD) in some, but not all, case-control studies. Neuroepidemiology. 1999;18:303–8. [Google Scholar]
- Hamilton RF, Jr, Thakur SA, Holian A. Silica binding and toxicity in alveolar macrophages. Free Radic Biol Med. 2008;44:1246–58. doi: 10.1016/j.freeradbiomed.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris WR, Wang Z, Hamada YZ. Competition between transferrin and the serum ligands citrate and phosphate for the binding of aluminum. Inorg Chem. 2003;42:3262–73. doi: 10.1021/ic026027w. [DOI] [PubMed] [Google Scholar]
- Ho A, Blum M. Induction of interleukin-1 associated with compensatory dopaminergic sprouting in the denervated striatum of young mice: model of aging and neurodegenerative disease. J Neurosci. 1998;18:5614–29. doi: 10.1523/JNEUROSCI.18-15-05614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson ET. Aluminum exposure and Alzheimer’s disease. J Alzheimers Dis. 2001;3:541–9. doi: 10.3233/jad-2001-3604. [DOI] [PubMed] [Google Scholar]
- Kausz AT, Antonsen JE, Hercz G, Pei Y, Weiss NS, Emerson S, Sherrard DJ. Screening plasma aluminum levels in relation to aluminum bone disease among asymptomatic dialysis patients. Am J Kidney Dis. 1999;34:688–93. doi: 10.1016/S0272-6386(99)70394-X. [DOI] [PubMed] [Google Scholar]
- Kawahara M. Effects of aluminum on the nervous system and its possible link with neurodegenerative diseases. J Alzheimers Dis. 2005;8:171–82. doi: 10.3233/jad-2005-8210. [DOI] [PubMed] [Google Scholar]
- Kriz J. Inflammation in ischemic brain injury: timing is important. Crit Rev Neurobiol. 2006;18:145–57. doi: 10.1615/critrevneurobiol.v18.i1-2.150. [DOI] [PubMed] [Google Scholar]
- Lazerte BD, Van Loon G, Anderson B. Aluminum in water. In: Yokel RA, Golub MS, editors. Research Issues in Aluminum Toxicity. Taylor and Francis; Washington: 1997. pp. 17–46. [Google Scholar]
- Li H, Campbell A, Ali SF, Cong P, Bondy SC. Chronic exposure to low levels of aluminum alters cerebral cell signaling in response to acute MPTP treatment. Toxicol Indust Health. 2008;23:515–524. doi: 10.1177/0748233708089027. [DOI] [PubMed] [Google Scholar]
- McLachlan DRC, Dalton AJ, Kruck TPA, Bell MY, Smith WL, Kalow W, Andrews DF. Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet. 1991;337:1304–8. doi: 10.1016/0140-6736(91)92978-b. [DOI] [PubMed] [Google Scholar]
- McLachlan DRC, Bergeron C, Smith JE, Boomer D, Rifat SL. Risk for neuropathologically confirmed Alzheimer’s disease and residual aluminum in municipal drinking water employing weighted residential histories. Neurology. 1996;46:401–5. doi: 10.1212/wnl.46.2.401. [DOI] [PubMed] [Google Scholar]
- Miu AC, Olteanu AI, Miclea M. A behavioral and ultrastructural dissection of the interference of aluminum with aging. J. Alzheimers Dis. 2004;6:315–28. doi: 10.3233/jad-2004-6312. [DOI] [PubMed] [Google Scholar]
- Mrak RE, Sheng JG, Griffin WST. Glial cytokines in Alzheimer’s Disease: review and pathogenic implications. Human Pathol. 1995;26:816–23. doi: 10.1016/0046-8177(95)90001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharmaceut Desig. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Nieboer E, Gibson BL, Oxman AD, Kramer JR. Health Effects of Aluminum: A Critical Review with Emphasis on Aluminum in Drinking Water. Environ Rev. 1995;3:29–81. [Google Scholar]
- Ookubo M, Yokoyama H, Kato H, Araki T. Gender differences on MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) neurotoxicity in C57BL/6 mice. Mol Cell Endocrinol. 311:62–8. doi: 10.1016/j.mce.2009.07.011. 200. [DOI] [PubMed] [Google Scholar]
- Perl DP, Gajdusek DC, Garruto RM, Yangihara RT, Gibbs CJ. Intraneuronal accumulation of aluminum in amyotropic lateral sclerosis and Parkinsonism-dementia of Guam. Science. 1982;217:1053–9. doi: 10.1126/science.7112111. [DOI] [PubMed] [Google Scholar]
- Perreau VM, Cotman CW, Sharman KG, Bondy SC, Sharman EH. Melatonin treatment in old mice enables a more youthful response to LPS in the brain. J Neuroimmunol. 2007;182:22–31. doi: 10.1016/j.jneuroim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrik MS, Wong MC, Tabata RC, Garry RF, Shaw CA. Aluminum adjuvant linked to Gulf War illness induces motor neuron death in mice. Neuromolecular Med. 2007;9:83–100. doi: 10.1385/nmm:9:1:83. [DOI] [PubMed] [Google Scholar]
- Phelps KR, Naylor K, Brien TP, Wilbur H, Haqqie SS. Encephalopathy after bladder irrigation with alum: Case report and literature review. Amer J Med Sci. 1999;318:181–5. doi: 10.1097/00000441-199909000-00013. [DOI] [PubMed] [Google Scholar]
- Priest ND, Talbot RJ, Newton D, Day JP, King SJ, Fifield LK. Uptake by man of aluminium in a public water supply. Hum Exp Toxicol. 1988;17:296–301. doi: 10.1177/096032719801700602. [DOI] [PubMed] [Google Scholar]
- Pratico D, Uryu K, Sung S, Tang S, Trojanowski JQ, Lee VM. Aluminum modulates brain amyloidosis through oxidative stress in APP transgenic mice. FASEB J. 2002;16:1138–40. doi: 10.1096/fj.02-0012fje. [DOI] [PubMed] [Google Scholar]
- Polizzi S, Pira E, Ferrara M, Bugiani M, Papaleo A, Albera R, Palmi S. Neurotoxic effects of aluminium among foundry workers and Alzheimer’s disease. Neurotoxicology. 2002;23:761–74. doi: 10.1016/S0161-813X(02)00097-9. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–62. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF. Relation between aluminum concentrations in drinking water and Alzheimer’s disease: an 8-year follow-up study. Amer J Epidemiol. 2000;152:59–66. doi: 10.1093/aje/152.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau V, Jacqmin-Gadda H, Commenges D, Helmer C, Dartigues JF. Aluminum and silica in drinking water and the risk of Alzheimer’s disease or cognitive decline: findings from 15-year follow-up of the PAQUID cohort. Am J Epidemiol. 2009;169:489–96. doi: 10.1093/aje/kwn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo LS, Beale G, Sandroni S, Ballinger WE. Aluminium intoxication in undialysed adults with chronic renal failure. J Neurol Neurosurg Psychiatry. 1992;155:697–700. doi: 10.1136/jnnp.55.8.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selley ML. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005;1037:1–6. doi: 10.1016/j.brainres.2004.02.083. [DOI] [PubMed] [Google Scholar]
- Sharman KG, Sharman E, Bondy SC. Dietary melatonin selectively reverses age-related changes in cortical basal cytokine mRNA levels, and their responses to an inflammatory stimulus. Neurobiol Aging. 2002;23:633–8. doi: 10.1016/s0197-4580(01)00329-3. [DOI] [PubMed] [Google Scholar]
- Sharman E, Sharman KG, Lahiri DK, Bondy SC. Age-related changes in murine CNS mRNA gene expression are modulated by dietary melatonin. J Pineal Res. 2004;36:165–70. doi: 10.1046/j.1600-079x.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- Sharman EH, Sharman KG, Cotman CW, Bondy SC, Lahiri DK, Perreau VM. Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochem Int. 2007;50:336–44. doi: 10.1016/j.neuint.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard DJ, Walker JV, Boykin JL. Precipitation of dialysis dementia by deferoxamine treatment of aluminum-related bone disease. Am J Kidney Dis. 1988;12:126–30. doi: 10.1016/s0272-6386(88)80007-6. [DOI] [PubMed] [Google Scholar]
- Shigematsu K, McGeer PL. Accumulation of amyloid precursor protein in damaged neuronal processes and microglia following intracerebral administration of aluminum salts. Brain Res. 1992;593:117–23. doi: 10.1016/0006-8993(92)91272-g. [DOI] [PubMed] [Google Scholar]
- Shin RW, Kruck TP, Murayama H, Kitamoto T. A novel trivalent cation chelator Feralex dissociates binding of aluminum and iron associated with hyperphosphorylated tau of Alzheimer’s disease. Brain Res. 2003;961:139–46. doi: 10.1016/s0006-8993(02)03893-3. [DOI] [PubMed] [Google Scholar]
- Shirabe T, Irie K, Uchida M. Autopsy case of aluminum encephalopathy. Neuropathology. 2002;22:206–10. doi: 10.1046/j.1440-1789.2002.00432.x. [DOI] [PubMed] [Google Scholar]
- Smith RW. Kinetic aspects of aqueous aluminum chemistry: environmental implications. Coord Chem Rev. 1996;149:81–93. [Google Scholar]
- Sparkman NL, Johnson RW. Neuroinflammation associated with aging sensitizes the brain to the effects of infection or stress. Neuroimmunomodulation. 2008;15:323–30. doi: 10.1159/000156474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparling DW, Campbell PGC. Ecotoxicology of aluminum to fish and wildlife. In: Yokel RA, Golub MS, editors. Research Issues in Aluminum Toxicity. Taylor and Francis; Washington: 1997. pp. 48–68. [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Styren SD, Kamboh MI, Dekosky ST. Expression of differential immune factors in temporal cortex and cerebellum: the role of α-1-antichymotrypsin, Apolipoprotein E, and reactive glia in the progression of Alzheimer’s Disease. J Comp Neurol. 1998;396:511–20. [PubMed] [Google Scholar]
- Sun YX, Minthon L, Wallmark A, Warkentin S, Blennow K, Janciauskiene S. Inflammatory markers in matched plasma and cerebrospinal fluid from patients with Alzheimer’s Disease. Dement. Geriatr Cogn Disord. 2003;16:136–44. doi: 10.1159/000071001. [DOI] [PubMed] [Google Scholar]
- Walton JR. Functional impairment in aged rats chronically exposed to human range dietary aluminum equivalents. Neurotoxicology. 2009;30:182–93. doi: 10.1016/j.neuro.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Yang EY, Guo-Ross SX, Bondy SC. The stabilization of ferrous iron by a toxic β-amyloid fragment and by an aluminum salt. Brain Res. 1999;799:91–6. doi: 10.1016/s0006-8993(99)01694-7. [DOI] [PubMed] [Google Scholar]
- Xu N, Majidi V, Markesbery WR, Ehmann WD. Brain aluminum in Alzheimer’s disease using an improved GFAAS method. Neurotoxicology. 1992;13:735–43. [PubMed] [Google Scholar]
- Yasui M, Kihira T, Ota K. Calcium, magnesium and aluminum concentrations in Parkinson’s disease. Neurotoxicology. 1992;13:593–600. [PubMed] [Google Scholar]
- Yokel RA, Allen DD, Ackley DC. The distribution of aluminum into and out of the brain. J Inorg Biochem. 1999;76:127–32. doi: 10.1016/s0162-0134(99)00124-5. [DOI] [PubMed] [Google Scholar]
- Yokel RA, Rhineheimer SS, Sharma P, Elmore D, McNamara PJ. Entry, half-life, and desferrioxamine-accelerated clearance of brain aluminum after a single (26)Al exposure. Toxicol Sci. 2001;64:77–82. doi: 10.1093/toxsci/64.1.77. [DOI] [PubMed] [Google Scholar]
- Zhao M, Cribbs DH, Anderson AJ, Cummings BJ, Su JH, Wasserman AJ, Cotman CW. The induction of the TNF alpha death domain signaling pathway in Alzheimer’s disease brain. Neurochem Res. 2003;28:307–18. doi: 10.1023/a:1022337519035. [DOI] [PubMed] [Google Scholar]