Abstract
We herein report the case of a 20-year-old man who underwent a photorefractive keratectomy (PRK). We measured matrix metalloproteinase-9 (MMP-9) activity and chondroitin 4 sulfate and chondroitin 6 sulfate concentrations in tear fluid. Tear fluid was collected pre-operatively via microcapillary tube, and was collected postoperatively on the first and fourth days, and after one week, one month, three months, and six months. Samples were formulated by dilution with 200 μL of saline. MMP-9 activity was analyzed by an enzyme immunocapture activity assay, and the concentrations of chondroitin sulfate were analyzed by enzyme-linked immunosorbent assay. No complications were observed after surgery, except for a minimal subepithelial haze. Although MMP-9 activity changed on the fourth postoperative day, the activity changed only minimally at this time. Chondroitin 4 sulfate concentrations in tear fluid increased dramatically from one week to one month, decreased transiently at three months, and increased by six months. The chondroitin 6 sulfate concentration did not normalize within one week, and decreased from one week to three months compared with the preoperative score, and was close to the preoperative score at six months. We conclude that corneal wound healing was still incomplete six months after PRK, and chondroitin 4 sulfate appears to be critical in this process.
Keywords: matrix metalloproteinase, chondroitin sulfate, human tear fluid, photorefractive keratectomy, corneal wound healing
Introduction
Matrix metalloproteinases (MMPs) are proteases that degrade collagen, a basement membrane constituent, and other components in the extracellular matrix.1 MMPs are important in tissue remodeling and wound healing.2 Twenty-eight types of MMPs are identified today. MMP-9 degrades collagens, fibronectin, elastin, and gelatins.3
Chondroitin sulfate is a component of the extracellular matrix that participates in tissue and wound healing in various organs, and has isomers containing sulfate, chondroitin 4 sulfate (C4S), and chondroitin 6 sulfate (C6S).4–6 Chondroitin sulfate is assumed to have a connection with matrix metalloproteinases (MMPs) because chondroitin sulfate affects the expression and activation of MMP in vitro,7 which decomposes the extracellular matrix.1
The use of laser refractive surgery has increased recently, and has become a subject of study as a model of corneal wound healing because it necessitates injury to the cornea.4,8–11 Photorefractive keratectomy (PRK) is a type of laser refractive surgery, and tends to induce a haze after surgery. A large amount of extracellular matrix accumulates in the haze on rabbit cornea.8 Holopainen et al have reported alteration in MMP-8 levels in tear fluid before and after PRK.10 Based on these findings, we collected tear fluid from the eyes of a patient who underwent PRK and measured MMP-9 activity and chondroitin sulfate concentrations in both eyes using an enzyme immunocapture activity assay (EIA) and an enzyme-linked immunosorbent assay (ELISA), respectively.
Case report
A 20-year-old man was referred to our institution to correct myopic astigmatism. The patient’s uncorrected visual acuity was 0.03 in the right eye and 0.09 in the left eye. The patient’s best corrected visual acuity was 2.0 (corrected with −7.50 D sph) in the right eye and 2.0 (corrected with −6.50 D sph −0.50 D cyl, axis 170°) in the left eye. The intraocular pressure was 13 mmHg in the right eye and 11 mmHg in the left eye. No abnormalities were found in the anterior segment of the eye, ocular media, or ocular fundus. PRK was planned because the patient wished to become a boxer. Informed consent was obtained from the patient after he received a detailed verbal explanation of the study prior to tear sample collection, in accordance with the 2000 World Medical Association Declaration of Helsinki.
Photorefractive keratectomy
Simultaneous binocular PRK was performed for emmetropia. The VISXR Star S3 excimer laser (VISX®, USA Inc.; wavelength 193 nm, fluence 160 mJ/cm2, and pulse rate 10 Hz) was used. The eye tracking system in the excimer laser was active during the procedure. The planned laser ablation was performed on the Bowman membrane and corneal stroma after surgical ablation of the epithelium. The ablation diameter was 6.5 mm, and the ablation depth was 108 μm in the right eye and 96 μm in the left eye.
The surgical field was washed using 0.3 mM oxyglutathione solution after laser ablation, and disposable soft contact lenses were worn in both eyes. No complications were observed during surgery. After the procedure, 0.5% levofloxacin and betamethasone sodium phosphate 0.1% were administered into the eyes.
Postoperative course
The patients received levofloxacin 0.5%, fluorometholone 0.1%, and sodium hyaluronate four times daily for one week after surgery. One week later, levofloxacin 0.5% and fluorometholone 0.1% were tapered to twice daily and discontinued one month postoperatively, and sodium hyaluronate 0.3% was applied as needed. Four days later, the contact lenses were removed from the eyes. Although postoperative minimal haze was observed after one month, no effect was observed on the patient’s visual acuity or refraction. The patient’s uncorrected bilateral visual acuity was 1.5 and his best corrected bilateral visual acuity was 2.0 (corrected with −0.50 D sph) at six months postoperatively.
Methods
Samples
Tear fluid was collected from the lower conjunctival sac with a microcapillary tube (Minicaps, Hirschmann Laborgerate, capillary length 32 mm, maximum volume 0.5 μL). To prevent conjunctival stimulation and reflex tearing, care was taken not to touch the conjunctiva. The collection time was 30 seconds. When the capillary filled with tear fluid within 30 seconds, additional capillaries were applied immediately to the conjunctival sac until 30 seconds had elapsed. No topical anesthesia was applied during the collection process, and no topical agents were used in the 30 minutes prior to collection of the tear fluid. The tear volume was calculated by measuring the height of the tear fluid in the microcapillary tube. We collected 0.3–3.0 μL of tear fluid from each eye and formulated the samples by diluting the collected tear fluid with 200 μL of saline. The samples were frozen immediately at −40°C after collection until the measurements were performed. Tear fluid was collected preoperatively and then postoperatively on the first and fourth days, and at the end of the first week, and at one, three, and six months. All samples were collected by the same investigator.
MMP-9 activity and chondroitin sulfate levels
The activity of MMP-9 in the tear fluid was measured by EIA and the concentrations of each chondroitin sulfate isomer were measured by a sandwich ELISA, which we have previously reported.4,14 We describe the main aspects of each measurement method below.
MMP-9 is produced as a pro-MMP-9 inside the cell. It is released into the extracellular space and converted to active MMP-9, which decomposes the extracellular matrix. Only active-type MMP-9 activity can be measured by EIA.
The sandwich ELISA method was modified to improve the accuracy of measuring low chondroitin sulfate. The standard chondroitin sulfate solution used in the ELISA was comprised of heterogeneous polysaccharides, which were extracted from dogfish cartilage and purified. Antibodies to C4S and C6S were used as the primary antibodies. The epitope of these antibodies is a 4- to 5-polysaccharide chain in chondroitin sulfate. Chondroitin sulfates with more than 8- to 10-polysaccharide chains in the tear fluid can be detected in the sandwich ELISA.
Results
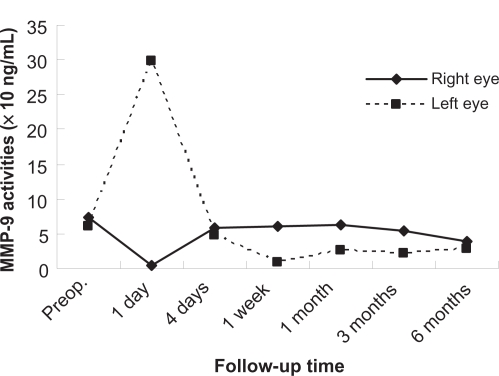
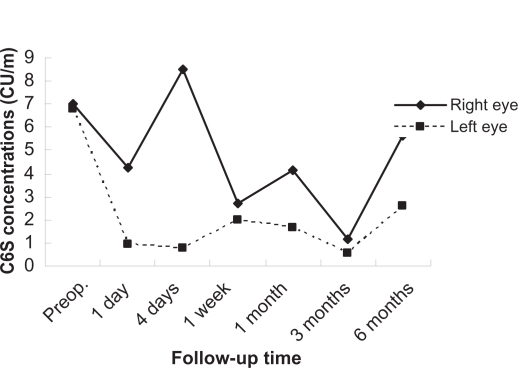
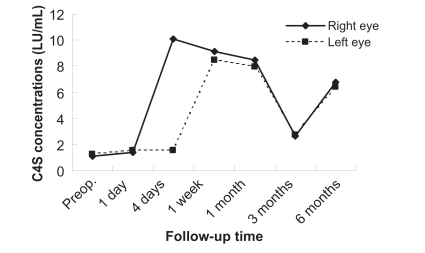
Figures 1 to 3 show the postoperative changes in MMP-9 activity and chondroitin sulfate concentrations, respectively. MMP-9 activity did not renormalize within four days, and was changed slightly at this time compared with preoperative levels. The C4S concentration increased dramatically from the first week to one month, was decreased at three months, and increased at six months. The C6S concentration did not normalize within one week, decreased from the first week to three months from the preoperative score, and got closer to the preoperative score at six months, but did not actually reach it.
Figure 1.
Changes in matrix metalloproteinase-9 in tear fluid after photorefractive keratectomy. The vertical axis represents the matrix metalloproteinase-9 activity, and the horizontal axis represents time.
Figure 3.
Changes in chondroitin 6 sulfate in tear fluid after photorefractive keratectomy. The vertical axis represents the chondroitin 6 sulfate concentration, the horizontal axis is time.
Discussion
Although MMP-9 activity in the tear fluid was changed on the first postoperative day, the levels were changed only minimally from the preoperative value after four days. MMP-9 may participate in corneal wound healing shortly after PRK, whereas the MMP-8 concentrations have been found to be significantly higher in patients undergoing PRK than in control subjects on the second postoperative day.10 This may indicate that MMP-8 and MMP-9 participate in different phases of corneal wound healing. The changes in the right and left eye were different on the first postoperative day. We will need to perform more of these procedures to clarify this finding.
In contrast, the C4S concentration in the tear fluid changed dramatically. These changes indicated that C4S can participate greatly in corneal wound healing following PRK. However, Oya et al have reported that C4S did not show any significant variation in tear fluid in a rabbit model of wound healing.5 We performed PRK on the human cornea with an excimer laser, but Oya et al performed a scrape on the rabbit corneal epithelium with a trephine and spatula.5 The present study differs from the report by Oya et al5 in the use of materials and surgical procedures. Nakayasu et al have reported that the wound healing process was still incomplete three years after PRK in rabbits.9 Increased C4S concentrations six months postoperatively indicate that the wound healing process was still incomplete. The C6S concentration in the tear fluid was still abnormal at one week, and levels decreased from the first week to three months following the preoperative collection. These changes indicate that C6S seems to be implicated in corneal wound healing following PRK, and that low C6S levels activate the extracellular matrix, and can affect corneal wound healing as a result.
Contact lenses affect the ocular surface.11 Disposable soft contact lenses worn for four days following PRK might therefore affect tear secretion, tear exchange, and tear distribution.11 These changes might affect MMP-9 activity and concentrations during corneal wound healing. The administration of topical corticosteroids following PRK is another factor that affects corneal wound healing. However, the stability of refraction and the occurrence of haze are not frequently observed.12,13 Therefore, topical corticosteroids do not seem to play a beneficial role in MMP-9 activity and chondroitin sulfate concentration in corneal wound healing.
MMPs are inhibited by naturally-occurring proteins, tissue inhibitors of MMPs, and by the broad-spectrum inhibitor, α2-macroglobulin.1 MMPs are synthesized primarily by connective tissue cells, granulocytes, and monocyte macrophages,1 and are present in various body fluids, including tears.1,2 The tear fluid is formed from the lipid layer, aqueous layer, and mucous layer. It also shields the cornea from the environment, and is essential for corneal transparency.4 MMP-9, C4S, and C6S are present in normal human tear fluid.1,4,6,14,15 During corneal wound healing, MMP contributes to the synthesis and decomposition of the extracellular matrix.16 Based on these findings, MMPs will interact with factors such as tissue inhibitors of MMPs, the extracellular matrix, and chondroitin sulfate, which are well balanced and participate actively in the corneal wound healing process.
We conclude that corneal wound healing was still incomplete six months after PRK, and C4S in tear fluid may participate in corneal wound healing after PRK in particular. The changes in MMP-9 activity and chondroitin sulfate concentrations may be correlated with problems following PRK, such as visual haze caused by a large amount of extracellular matrix deposits. To reduce these complications, further studies will examine MMP-9 and chondroitin sulfate levels in tear fluid in the future.
Figure 2.
Changes in chondroitin 4 sulfate in tear fluid after photorefractive keratectomy. The vertical axis represents the chondroitin 4 sulfate concentration, the horizontal axis is time.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Leonardi A, Brun P, Abatangelo G, et al. Tear levels and activity of matrix metalloproteinase (MMP)-1 and MMP-9 in vernal keratoconjunctivitis. Invest Ophthalmol Vis Sci. 2003;44:3052–3058. doi: 10.1167/iovs.02-0766. [DOI] [PubMed] [Google Scholar]
- 2.Hanemaaijer R, Visser H, Konttinen YT, et al. A novel and simple immunocapture assay for determination of gelatinase-B (MMP-9) activities in biological fluids: Saliva from patients with Sjögren’s syndrome contain increased latent and active gelatinase-B levels. Matrix Biol. 1998;17:657–665. doi: 10.1016/s0945-053x(98)90116-0. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi T. Role of matrix metalloproteinase-9 expression on cutaneous inflammation: Possible treatment by leptomycin B application. Inflammation and Regeneration. 2004;24:578–583. [Google Scholar]
- 4.Nishio M, Arai K. Quantitative analysis of chondroitin sulfate in tear fluids following laser in situ keratomileusis. Ophthalmic Res. 2006;38:274–279. doi: 10.1159/000095770. [DOI] [PubMed] [Google Scholar]
- 5.Oya T, Obata H, Miyata K, et al. Quantitative analysis of glycosaminoglycans in tear fluids during corneal epithelial wound healing in rabbits. Jpn J Ophthalmol Soc. 1994;98:1049–1055. [PubMed] [Google Scholar]
- 6.Oya T, Obata H, Miyata K, et al. Quantitative analysis of glycosaminoglycans in tear fluids in normal human eyes and eyes with corneal epithelial disorders. Jpn J Ophthalmol Soc. 1995;99:302–307. [PubMed] [Google Scholar]
- 7.Isnard N, Robert L, Renard G. Effect of sulfated GAGs on the expression and activation of MMP-2 and MMP-9 in corneal and dermal explant cultures. Cell Biol Int. 2003;27:779–784. doi: 10.1016/s1065-6995(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 8.Nakayasu K, Gotoh T, Ishikawa T, et al. Glycosaminoglycans in sub-epithelial opacity after excimer laser keratectomy. Jpn J Ophthalmol Soc. 1996;100:350–357. [PubMed] [Google Scholar]
- 9.Nakayasu K, Watanabe Y, Gotoh T, et al. Long-term healing of excimer laser ablated rabbit corneas. Jpn J Ophthalmol Soc. 1999;103:99–107. [PubMed] [Google Scholar]
- 10.Holopainen JM, Moilanen JAO, Sorsa T, et al. Activation of matrix metalloproteinase-8 by membrane type 1-MMP and their expression in human tears after photorefractive keratectomy. Invest Ophthalmol Vis Sci. 2003;44:2550–2556. doi: 10.1167/iovs.02-1190. [DOI] [PubMed] [Google Scholar]
- 11.Kinoshita S, Maruyama K, Komuro A, et al. The ocular surface and contact lens wear. J Jpn CL Soc. 2003;45:2–10. [Google Scholar]
- 12.Corbett MC, O’Brart DP, Marshall J. Do topical corticosteroids have a role following excimer laser photorefractive keratectomy? J Refract Surg. 1995;11:380–387. doi: 10.3928/1081-597X-19950901-15. [DOI] [PubMed] [Google Scholar]
- 13.Baek SH, Chang JH, Choi SY, et al. The effect of topical corticosteroids on refractive outcome and corneal haze after photorefractive keratectomy. J Refract Surg. 1997;13:644–652. doi: 10.3928/1081-597X-19971101-11. [DOI] [PubMed] [Google Scholar]
- 14.Mutoh T, Nishio M, Matsumoto Y, et al. Correlation between the matrix metalloproteinase-9 activity and chondroitin sulfate concentrations in tear fluid after laser in situ keratomileusis. Clin Ophthalmol. (In press). [DOI] [PMC free article] [PubMed]
- 15.Sobrin L, Liu Z, Monroy DC, et al. Regulation of MMP-9 activity in human tear fluid and corneal epithelial culture supernatant. Invest Ophthalmol Vis Sci. 2000;41:1703–1709. [PubMed] [Google Scholar]
- 16.Matsubara M, Girard MT, Kublin CL, et al. Differential roles for two gelatinolytic enzymes of the matrix metalloproteinase family in the remodeling cornea. Dev Biol. 1991;147:425–439. doi: 10.1016/0012-1606(91)90300-r. [DOI] [PubMed] [Google Scholar]