Abstract
The inhibitor of apoptosis proteins (IAPs) have recently been shown to modulate nuclear factor κB (NF-κB) signaling downstream of tumor necrosis factor (TNF) family receptors, positioning them as essential survival factors in several cancer cell lines, as indicated by the cytotoxic activity of several novel small molecule IAP antagonists. In addition to roles in cancer, increasing evidence suggests that IAPs have an important function in immunity; however, the impact of IAP antagonists on antitumor immune responses is unknown. In this study, we examine the consequences of IAP antagonism on T cell function in vitro and in the context of a tumor vaccine in vivo. We find that IAP antagonists can augment human and mouse T cell responses to physiologically relevant stimuli. The activity of IAP antagonists depends on the activation of NF-κB2 signaling, a mechanism paralleling that responsible for the cytotoxic activity in cancer cells. We further show that IAP antagonists can augment both prophylactic and therapeutic antitumor vaccines in vivo. These findings indicate an important role for the IAPs in regulating T cell–dependent responses and suggest that targeting IAPs using small molecule antagonists may be a strategy for developing novel immunomodulating therapies against cancer.
The inhibitor of apoptosis proteins (IAPs) were initially identified as caspase inhibitors capable of blocking both extrinsic and intrinsic apoptotic signals. Recent work has established diverse roles for the IAP family, in which they have been shown to regulate apoptosis through the modulation of NF-κB signaling downstream of several TNF family receptors and to play an essential role in the modulation of FAS-induced cell death (Hu et al., 2006; Leulier et al., 2006; Rigaud et al., 2006; Gaither et al., 2007; Lu et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007, 2008; Vince et al., 2007, 2008; Xu et al., 2007; Bertrand et al., 2008; Mahoney et al., 2008; Matsuzawa et al., 2008; Srinivasula and Ashwell, 2008; Wang et al., 2008; Csomos et al., 2009; Jost et al., 2009). All IAPs contain baculovirus inhibitory repeat domains that mediate protein binding, and several, including cellular IAP-1 (cIAP-1) and cIAP-2, X-linked IAP (XIAP), and melanoma-IAP/Livin, contain RING finger E3 ubiquitin ligase domains, which can cause autoubiquitination as a means of regulating apoptosis (Schile et al., 2008; Srinivasula and Ashwell, 2008). IAPs are regulated endogenously by second mitochondrial-derived activator of caspases (SMAC), which interacts with IAP baculovirus inhibitory repeat domains via a tetrapeptide motif. Several pharmacologic SMAC mimetics have been developed that induce tumor death through binding to the RING domain containing IAPs and leading to ubiquitin-mediated destruction (Gaither et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007; Vince et al., 2007; Wang et al., 2008). These pharmacologic SMAC mimetics act as broad antagonists of the RING domain containing IAPs and are actively being investigated as a potential novel class of cancer chemotherapeutics.
In addition to roles in tumor biology, several studies suggest important functions for the IAPs in immunoregulation. XIAP-deficient humans develop X-linked lymphoproliferative disease and were initially reported to lack NKT cells, although the specificity of this finding has recently been challenged (Rigaud et al., 2006; Marsh et al., 2009). XIAP-deficient mice have difficulty controlling Listeria monocytogenes infections and are more susceptible to infection with MHV-68 (mouse herpes virus 68); however, the mechanism for this immunodeficiency is unknown and is not associated with decreased NKT cell function (Bauler et al., 2008; Rumble et al., 2009). cIAP-2 is involved in a recurrent translocation in mucosal-associated lymphoid tissue lymphoma and has been reported to function as an E3 ligase for BCL10 in lymphocytes, although the physiological importance of this activity is unknown (Hu et al., 2006). More recently, the cIAPs were shown to be critical for c-Jun N-terminal kinase activation downstream of CD40 and to negatively regulate alternative NF-κB activation by the BAFF (B cell activation factor of the TNF family) receptor (Matsuzawa et al., 2008; Vallabhapurapu et al., 2008; Zarnegar et al., 2008). These findings position the cIAPs as potentially key regulators of B cell homeostasis, although how the cIAPs regulate B cell–dependent immune responses has, at present, been incompletely explored. In addition to roles in adaptive immunity, the cIAPs and XIAP have been shown to be required for NOD-1 and -2 (nucleotide biding and oligomerization domain 1 and 2) signaling and downstream cytokine production after exposure to muramyl dipeptide (Bertrand et al., 2009; Krieg et al., 2009). Furthermore, cIAP-2–deficient mice show altered responses to lipopolysaccharide that may indicate a role for cIAP-2 in inflammatory cytokine-induced apoptosis in macrophages (Conte et al., 2006). Moreover, neuronal apoptosis inhibitor protein (NAIP), a member of both the NOD-like receptor and IAP families, is a component of the inflammasome and is required for control of Legionella pneumophila infections (Diez et al., 2003; Rigaud et al., 2006).
Although evidence now links the IAP family to regulation of both tumor cell survival and immune function, the impact of IAP inhibitors on antitumor immune responses is unknown. In particular, the consequences of IAP antagonism in the key effector cells responsible for antitumor immunity such as CD4+ and CD8+ T cells, NKT cells, and NK cells has not been explored. Given the potential for IAP antagonists to simultaneously induce tumor cell death and modulate immunity, understanding how IAP antagonism might alter nascent antitumor responses and responses to other forms of tumor immunotherapy may have implications for the use of these agents to treat cancer.
In this study, we examine the consequences of IAP antagonism on T cell function both in vitro and in the context of a tumor vaccine in vivo. Unexpectedly, we find that IAPs function as negative co-stimulators during T cell stimulation and that small molecule IAP antagonists can augment both human and mouse T cell responses to physiologically relevant stimuli, including tumor antigens, without producing responses in unstimulated cells.
RESULTS
IAP antagonists have co-stimulatory activity in effector T cells
Effector T cells play a critical role in antitumor immunity. Consequently, the effect of IAP antagonism on T cell function could impact antitumor immune responses occurring in the context of IAP antagonist–mediated tumor cell death. To investigate how IAP antagonists may influence T cell function, we exposed CD4+ T cells isolated from mouse spleens to several IAP antagonists (M1/LBW-242, M2, and M3) or to a control compound of similar structure (C1/LCV-843; Gaither et al., 2007). We found no evidence of enhanced apoptosis in IAP antagonist–treated T cells regardless of whether the cells were left unstimulated or received a polyclonal-activating signal using antibodies directed against CD3 and CD28 (Fig. 1 A and not depicted). Furthermore, in contrast to tumor cells that are sensitive to IAP antagonism, caspase 3 cleavage was not apparent in IAP antagonist–treated T cells (Fig. 1 B).
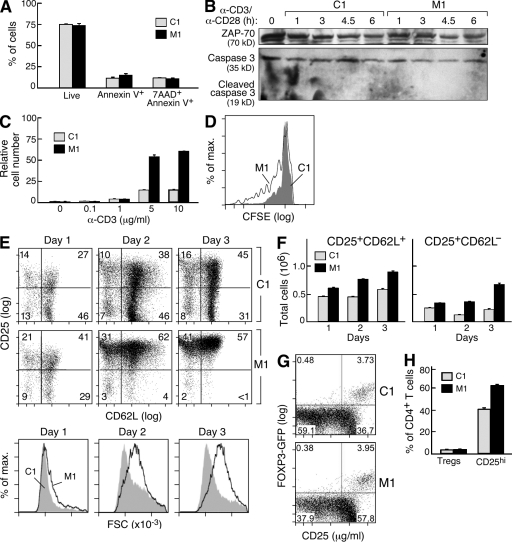
Figure 1.
IAP antagonists enhance mouse T cell proliferation and activation. (A–H) CD4+ T cells were positively selected from mouse spleens using magnetic beads and stimulated with 10 µg/ml plate-bound anti-CD3 (or as indicated) and 2 µg/ml anti-CD28 in the presence of IAP antagonist (M1) or control compound (C1) at 500 nM. (A) 5 × 105 CD4+ T cells were stimulated for 24 h. Annexin V and 7AAD staining were determined by flow cytometry. (B) Immunoblots for ZAP-70 and caspase 3 on total cell lysates from CD4+ T cells stimulated as indicated. (C and D) 105 CD4+ T cells were stimulated as indicated. (C) After 72 h, relative cell numbers were determined using CellTiter-Glo luminescent cell viability assay (Promega) and normalized to unstimulated cultures treated with C1. (D) Cells were labeled with CFSE before stimulation, and fluorescence was measured after 72 h by flow cytometry. (E) 5 × 105 CD4+ T cells were stimulated for the indicated periods of time, and CD25, CD62L, and forward scatter (FSC) were determined by flow cytometry. (F) Quantification of E using cell numbers determined by trypan blue exclusion. (G and H) 105 CD4+ T cells were isolated from the spleens of FOXP3-GFP knockin mice and stimulated for 72 h; CD25 and GFP were measured by flow cytometry. (H) Quantification of G using three replicates per group. (A–H) Error bars represent SEM. Results are representative of at least two independent experiments.
Although apoptosis was not affected, IAP antagonists did have a significant effect on T cell function, leading to a substantial increase in T cell numbers after 72 h of culture (P < 0.005 for 5 and 10 µg/ml; Fig. 1 C and Fig. S1). This effect was dependent on strong stimulation and was observed in both anti-CD3– and anti-CD3/CD28–stimulated cultures but not in cultures in which cells were left unstimulated or weakly stimulated (Fig. 1 C and Fig. S1). To determine whether this increase in cell number resulted from an effect on proliferation, we stained CD4+ T cells with the dye CFSE and stimulated them in the presence of IAP antagonists. After 3 d of stimulation, IAP antagonist–treated cultures showed substantially more CFSE dilution than controls, indicating increased proliferation (Fig. 1 D).
In addition to enhanced proliferation, T cells stimulated in the presence of IAP antagonists showed other signs of enhanced activation. IAP antagonist treatment of stimulated CD4+ T cells was associated with changes in surface marker expression characteristic of enhanced activation, including more rapid increases in CD25 and decreases in CD62L and an overall increase in cell size (Fig. 1 E). After 1 d of culture, stimulated T cells treated with M1 reached a level of activation comparable with control cells after 3 d and, in combination with proliferation, produced overall increases in total activated T cells (P < 0.005 for days 1–3; Fig. 1 F).
To exclude the possibility that increased numbers of CD4+CD25+ cells represented an increase in the T reg cell population, which has an overlapping surface phenotype with activated T cells (Fontenot et al., 2005), we stimulated CD4+ T cells isolated from mice expressing the transcription factor FOXP3 linked to GFP; FOXP3 is required for T reg cell development and maintenance, and in these mice, all T reg cells are marked with GFP (Fontenot et al., 2005). As was observed in wild-type animals, CD4+ T cells isolated from FOXP3-GFP mice showed increased CD25 expression upon stimulation in the presence of IAP antagonists; however, after 2 d in culture, the number of FOXP3-GFP–expressing cells remained essentially unchanged, demonstrating that IAP antagonism does not selectively expand this population (Fig. 1, G and H).
We next tested whether IAP antagonists could enhance cytokine production from stimulated CD4+ T cells in a manner similar to their effect on other markers of T cell activation. Consistent with enhanced activation, isolated CD4+ T cells treated with IAP antagonists and stimulated by increasing concentrations of anti-CD3 in the presence or absence of 2 µg/ml anti-CD28 produced substantially more IL-2 than similarly cultured cells treated with control compound (P < 0.005 for 5 and 10 µg/ml; Fig. 2 A). The effect of IAP antagonism was dose dependent and was observed with three distinct compounds (P = 0.0002 for M1; Fig. 2 B and Fig. S2). In addition to effects on IL-2 production, levels of both IL-4 and IFN-γ were increased in IAP antagonist–treated cultures (unpublished data). Isolated CD8+ T cells stimulated with anti-CD3/CD28 also responded to IAP antagonist treatment with an increase in cytokine production, indicating a general activation-enhancing effect of IAP antagonists on T cells (Fig. 2 C).
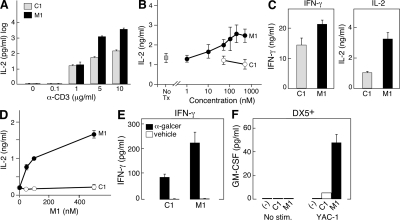
Figure 2.
IAP antagonists enhance the stimulation of multiple immune effectors. (A–C) CD4+ or CD8+ T cells were isolated as in Fig. 1. (A) 105 CD4+ T cells were isolated and stimulated with anti-CD3 as indicated and 2 µg/ml anti-CD28 for 72 h. M1 and C1 were used at 500 nM. (B) 105 CD4+ T cells were isolated and stimulated with 10 µg/ml anti-CD3 and 2 µg/ml anti-CD28 for 48 h in the presence of M1 or control compound at the indicated concentrations. (C) 105 CD8+ T cells were isolated and stimulated as in B for 48 h. M1 and C1 were used at 500 nM. (D) 105 lymph node cells from RAG-deficient OTI transgenic mice were stimulated for 72 h by 0.5% formaldehyde-fixed bone marrow–derived DCs that had been pulsed with 10 µM/ml OVA peptide (SIINFEKL) 4 h before fixation. M1 or a control compound was added to the media at the indicated concentrations. (E) 5 × 105 spleen cells were stimulated with 100 ng/ml α-galactosylceramide (α-galcer) or vehicle in the presence of M1 or control compound at 500 nM for 24 h. (F) 2 × 105 DX5+ NK cells were positively selected from mouse spleens using magnetic beads and stimulated for 48 h by co-culture with 4 × 104 YAC-1 cells in the presence of M1 or control compound at 500 nM. (A–F) Cytokines were measured by ELISA. Error bars represent SEM. Results are representative of at least two independent experiments.
T cells are activated physiologically by the recognition of antigenic peptides bound to MHC on the surface of antigen-presenting cells (Heemels and Ploegh, 1995). Consequently, we next sought to examine IAP antagonism in the context of peptide-restricted T cell activation. OTI CD8+ T cells, which recognize the OVA peptide SIINFEKL bound to MHC class I (Hogquist et al., 1994), were stimulated with peptide-loaded DCs in the presence of either the IAP antagonist M1 or control compound. As was observed in the context of activating antibodies, IAP antagonist treatment led to a dose-dependent enhancement in cytokine production with effects ranging from five- to sevenfold (P < 0.0001 for all M1 concentrations; Fig. 2 D); furthermore, IAP antagonists increased cytokine production at a range of peptide concentrations and when DCs were pulsed with whole OVA protein (Fig. S3; Hogquist et al., 1994).
In addition to peptide-reactive CD4+ and CD8+ T cells, both NKT cells and NK cells have been shown to play important roles in certain antitumor immune responses (Dougan and Dranoff, 2009). Consequently, we wondered how these cells types would respond to treatment with IAP antagonists. Consistent with the effect of IAP antagonists on peptide-specific responses, spleen cells stimulated with the NKT cell–specific agonist α-galactosylceramide produced significantly more IFN-γ than cells treated with control compound (P = 0.007; Fig. 2 E). This effect was specific to stimulated cultures, as no cytokine was produced by cultures treated with IAP antagonists alone (Fig. 2 E). NK cell responses were also sensitive to IAP antagonism, as IAP antagonist–treated NK cells produced substantially more granulocyte-macrophage CSF (GM-CSF) after exposure to NKG2D ligand expressing YAC-1 cells, although IAP antagonist treatment alone had no effect on GM-CSF production (P < 0.0001; Fig. 2 F; Raulet et al., 2001).
Collectively, these findings indicate that IAP antagonists can augment lymphocyte co-stimulation. As is the case with co-stimulatory signals delivered through surface receptors (Greenwald et al., 2005), in the absence of antigen stimulation, the IAP antagonists have no effect on any of the measured parameters of T cell activation, including proliferation, surface marker expression, and cytokine production; however, when T cells are given a strong activating signal, either with antibodies or peptide in the context of MHC, IAP antagonism leads to a significant enhancement in T cell function. Similar findings were observed with both NKT cells and NK cells, suggesting that IAP antagonists can broadly co-stimulate multiple cell types involved in antitumor responses and may be useful in augmenting antitumor immunity.
Human T cells are sensitive to IAP antagonists
We next decided to test whether our observations in mouse T cells could be generalized to human T cells. We first isolated human CD4+ T cells from the peripheral blood of healthy donors and stimulated them with anti-CD3/anti-CD28 in a manner analogous to our mouse T cell stimulations. Under these conditions, IAP antagonist treatment was associated with both dose-dependent enhancements in cytokine production (P < 0.0001; Fig. 3, A and B) and changes in surface marker expression and cell size (Fig. 3 C) similar to those observed with mouse cells. After 3 d of stimulation, activated CD25+CD62L− T cells represented nearly 29% of IAP antagonist–treated cells compared with 18% of control cells; however, in contrast to mouse cells, the total fraction of CD25+ T cells was only modestly increased (73.5 vs. 59.8%; Fig. 3 C). Also consistent with findings in the mouse, human cells were not activated by IAP antagonist treatment alone, further demonstrating a role for IAP antagonists in T cell co-stimulation (Fig. 3 A).
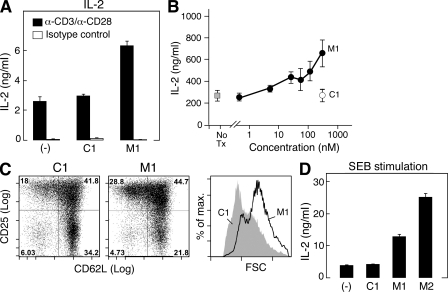
Figure 3.
Human T cells respond to IAP antagonists. (A–C) 105 human CD4+ T cells were isolated from the peripheral blood by positive selection using magnetic beads and stimulated with agonistic antibodies to 10 µg/ml anti-CD3 and 2 µg/ml anti-CD28 in the presence of M1 or a control compound at 500 nM (A and C) or as indicated (B). (A and B) IL-2 was measured after 48 h in the culture supernatant by ELISA. (C) Cells were stimulated for 72 h, and CD25, CD62L, and forward scatter (FSC) were measured by flow cytometry. (D) 2 × 105 human PBMCs were incubated with SEB for 96 h in the presence of 500 nM M1 or control compound. (A–D) Error bars represent SEM. Results are representative of at least three independent experiments.
We also assessed the ability to IAP antagonists to augment activation of T cells stimulated by the superantigen staphylococcus enterotoxin B (SEB), in addition to stimulation through activating antibodies. When human PBMCs were incubated with SEB, IAP antagonists enhanced cytokine production by as much as fivefold (P < 0.0001 for M1 and M2; Fig. 3 D). Collectively, these results indicate that IAP antagonist treatment can co-stimulate T cell activation in both mice and humans.
Alternative NF-κB signaling is required for IAP antagonist–induced T cell co-stimulation
The IAPs have been implicated in a wide range of signaling pathways that could modulate T cell activation (Srinivasula and Ashwell, 2008). Although caspases have a well-described role in T cell activation (Bidère et al., 2006) and several of the IAPs are known to regulate caspases, we found that relieving caspase inhibition does not appear to be the primary mechanism underlying IAP antagonist activity in T cells. In addition to failing to activate caspase 3, IAP antagonists had an equivalent effect on T cell activation regardless of whether the cells were pretreated with the caspase inhibitor ZVAD-fmk (Figs. 1 B and 4 A).
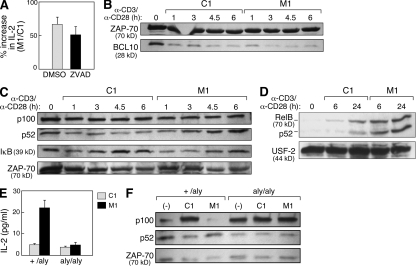
Figure 4.
IAP antagonists enhance T cell activation through the induction of alternative NF-κB signaling. (A–F) Mouse CD4+ T cells were isolated as in Fig. 1 or as indicated and stimulated with 10 µg/ml anti-CD3 and 2 µg/ml anti-CD28 in the presence of M1 or control compound at 500 nM. (A) CD4+ T cells were isolated and stimulated in the presence of the caspase inhibitor ZVAD-fmk or vehicle (DMSO). Data are presented as the ratio of IL-2 production measured in culture supernatants from M1-treated cells compared with control treatment. (B) Immunoblot for BCL10 in total cell lysates from stimulated CD4+ T cells. Lysates are identical to those depicted in Fig. 1 B. (C and D) Immunoblots using the indicated antibodies on total cell lysates (C) or purified nuclear lysates (D) from stimulated CD4+ T cells. (E) 105 naive T cells isolated from +/aly or aly/aly mouse spleens as depicted in Fig. S6 and stimulated in the presence of M1 or control compound. IL-2 was measured by ELISA. (A and E) Error bars represent SEM. (F) Immunoblots using the indicated antibodies on cell lysates from total +/aly or aly/aly CD4+ T cells isolated using magnetic beads and immediately lysed (−) or lysed after 24 h of stimulation (C1 and M1). (A–F) Results represent at least two independent experiments.
Because cIAP-2 has been reported to regulate NF-κB activation through the ubiquitination and degradation of BCL10 (Hu et al., 2006), we also assessed the effects of the IAP antagonists on BCL10 abundance. Although a decrease in BCL10 was observed upon T cell stimulation, this change was not altered by treatment with IAP antagonists, suggesting that the regulation of BCL10 abundance is not the principal mechanism of IAP antagonist activity in T cells (Fig. 4 B).
In tumor cells, IAP antagonist–mediated apoptosis depends on the modulation of both alternative and classical NF-κB signaling downstream of TNF family receptors. In these systems, the cIAPs constitutively down-regulate NF-κB–inducing kinase (NIK), blocking alternative NF-κB activation; however, the cIAPs are also indispensable in classical NF-κB activation, promoting the association of receptor-interacting protein with TAK1 (transforming growth factor β–activated kinase 1) through receptor-interacting protein ubiquitination (Gaither et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007, 2008; Vince et al., 2007, 2008; Mahoney et al., 2008; Wang et al., 2008). Regulation of TNF signaling by IAPs is conserved in Drosophila melanogaster, where DIAP2 (Drosophila IAP2) plays a critical role in the imd pathway, an immune response pathway orthologous to TNF signaling in mammals (Leulier et al., 2006).
Based on the evidence linking IAPs to NF-κB modulation, as well as the importance of NF-κB in T cell biology, we examined classical and alternative NF-κB signaling in T cells during IAP antagonist treatment (Karin and Lin, 2002). Stimulated mouse CD4+ T cells treated with M1 showed enhanced p100 processing to p52, as well as enhanced p52 and RelB nuclear localization (Fig. 4, C and D); in addition, M1 had moderate effects on IκB-α (inhibitor of NF-κB α) levels (Fig. 4 C). Activation of alternative NF-κB was also apparent in unstimulated T cells exposed to IAP antagonists, demonstrating that, similar to their effects in tumor cells, IAP antagonists are sufficient to activate alternative NF-κB in T cells even in the absence of antigen signaling (Fig. 5 B and Fig. S4).
Figure 5.
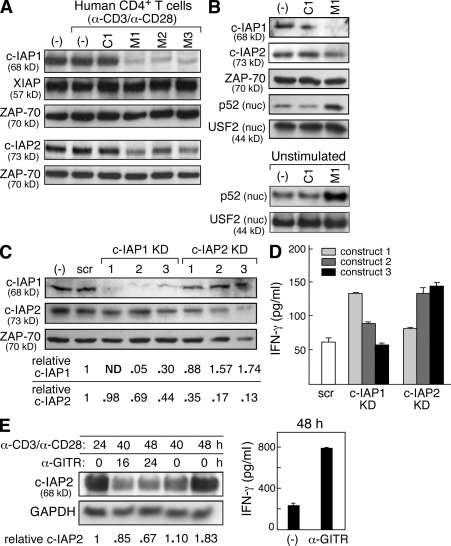
IAP antagonists target cIAP-1 and cIAP-2 in human T cells. (A and B) Immunoblots using the indicated antibodies on lysates from human CD4+ T cells isolated using magnetic beads. (A) Total lysates from CD4+ T cells were stimulated with 10 µg/ml anti-CD3 and 2 µg/ml anti-CD28 and exposed to IAP antagonists (M1, M2, and M3), a control compound (C1), or vehicle (−) for 2 h. All compounds were used at 500 nM. (B) Total cell lysates or nuclear (nuc.) lysates from human CD4+ T cells. Cells were either stimulated as in A (top) or left unstimulated (bottom) for 24 h in the presence of vehicle, M1, or control compound, which were used at 500 nM. (C and D) Human CD4+ T cells were isolated and stably transfected with lentiviral vectors encoding short hairpin RNAs against cIAP-1, cIAP-2, or nontargeting control (scramble [scr]). Three constructs (KD1–KD3) were used per gene. (C) Immunoblot using the indicated antibodies on lysates from primary CD4+ T cells expressing the indicated short hairpin RNA constructs; quantification of knockdown is included below. (D) 2 × 104 cells were stimulated for 72 h. 20 U/ml recombinant human IL-2 was added to all cultures. (E, left) Immunoblot using the indicated antibodies on total lysates from human CD4+ T cells stimulated with anti-CD3/CD28; after 24 h, cells were exposed to 5 µg/ml anti-GITR antibodies as indicated. (right) IFN-γ production from GITR-stimulated cells. (A–E) Cytokines were measured by ELISA. Error bars represent SEM. Results are representative of at least two independent experiments.
The observation of alternative NF-κB signaling in IAP antagonist–treated T cells suggests a role for this pathway in IAP antagonist–mediated co-stimulation. To directly test the importance of alternative NF-κB signaling in IAP antagonist–treated T cells, we evaluated responses to M1 in NIK-deficient alymphoplasia (aly/aly) mice (Miyawaki et al., 1994; Shinkura et al., 1999). Loss of NIK function prevents alternative NF-κB signaling, which has been shown to induce a suppressive phenotype in mature but not naive CD4+ T cells (Ishimaru et al., 2006); to avoid potentially confounding effects introduced by mixing naive and mature CD4+ T cells, we first isolated naive cells from aly/aly and aly/+ spleens using CD25 and CD44 expression (Fig. S5). Stimulation of naive aly/+ CD4+ T cells in the presence of IAP antagonists was associated with a substantial increase in IL-2 secretion compared with control treatment. In contrast, naive aly/aly CD4+ T cells were resistant to IAP antagonist treatment (Fig. 4 E). As expected, p100 processing to p52 after IAP antagonist treatment was also blocked in aly/aly CD4+ T cells (Fig. 4 F).
These results demonstrate that IAP antagonists require NIK to augment T cell activation, likely acting through alternative NF-κB signaling. Although this pathway is activated by IAP antagonists even in the absence of signaling through the antigen receptor, our results indicate that alternative NF-κB signaling alone cannot directly induce changes in T cell function. Signaling downstream of the antigen receptor likely activates complementary pathways that are necessary to enable IAP antagonists to effect a physiologically meaningful change in T cell function.
The cIAPs negatively regulate T cell activation
In principle, several IAPs could function as targets for IAP antagonists in T cells. Both human and mouse T cells express cIAP-1, cIAP-2, and XIAP (Yang and Li, 2000). Consistent with findings in a range of different cell types exposed to structurally diverse IAP antagonists (Gaither et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007; Vince et al., 2007; Wang et al., 2008), we observed decreases in cIAP-1 and cIAP-2 protein levels in human CD4+ T cells exposed to IAP antagonists, whereas XIAP levels remained constant (Fig. 5 A). Signaling through the T cell receptor was not required for cIAP reduction, as loss of the cIAPs occurred both in the presence and absence of stimulation (Fig. 5 A and Fig. S6). Consistent with our previous findings (Fig. 3 and Fig. 4, C–F) and other published work (Varfolomeev et al., 2007, 2008), degradation of the cIAPs in human T cells was associated with activation of alternative NF-κB signaling (Fig. 5 B); as was observed with mouse T cells, alternative NF-κB activation by IAP antagonists occurred in both stimulated and unstimulated cells (Fig. 5 B and Fig. S6).
To specifically address the role of the cIAPs in human T cells, we infected primary human CD4+ T cells with lentiviral constructs encoding short hairpin RNA directed against cIAP-1 and cIAP-2 (KD1–KD3; Moffat et al., 2006). Several of the evaluated constructs led to efficient knockdown of their targets in primary T cells (Fig. 5 C). In the mouse, loss of one cIAP leads to a compensatory increase in the other cIAP (Conze et al., 2005); however, transient knockdown of cIAP-1 and cIAP-2 in human T cells led to only a minor compensatory up-regulation of the other cIAP (Fig. 5 C). Efficient knockdown of either cIAP-1 or cIAP-2 in stimulated T cells was associated with increased IFN-γ production, suggesting a role of the cIAPs in negatively regulating activation (cIAP-1 KD1, P = 0.001; cIAP-2 KD2, P = 0.009; cIAP-2 KD3, P < 0.0001; Fig. 5 D).
cIAP-2 is regulated during T cell co-stimulation
These findings indicate a role for the cIAPs as regulators of T cell activation through their ability to negatively regulate alternative NF-κB signaling. Given recent evidence that TNF family ligands can induce cIAP-1 degradation downstream of their receptors (Varfolomeev et al., 2008), we hypothesized that the cIAPs may be regulated as part of T cell co-stimulation. Activated T cells express several TNF family co-stimulatory receptors that activate alternative NF-κB; among these, glucocorticoid-induced TNF receptor (GITR) is under active study in the context of tumor immunity, and thus we selected this molecule for more detailed characterization (Watts, 2005). Activation of human T cells with anti-CD3/CD28 led to cIAP-2 up-regulation; however, additional co-stimulation using anti-GITR antibodies was associated with a decrease in cIAP-2 concurrent with increased IFN-γ production and loss of p100 (Fig. 5 E and not depicted). Levels of cIAP-1 were not clearly changed in anti-GITR–stimulated cells during the time points examined (unpublished data). These findings suggest a physiological role for cIAP-2 downstream of GITR co-stimulation.
In vivo exposure to IAP antagonists enhances T cell susceptibility to stimulation but does not lead to generalized T cell activation
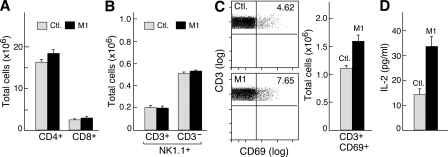
We next sought to evaluate the consequences of systemic delivery of the IAP antagonist M1 on T cell and NK cell populations in the spleen. Consistent with our findings in cell culture, in the absence of additional signaling, neither CD4+ nor CD8+ T cell numbers were altered by M1 administration, nor were any clear effects observed on NK cells or NK1.1+ T cells (Fig. 6, A and B). Although, as anticipated, broad T cell activation did not occur when mice were treated with M1, a small increase in the baseline number of CD69+ T cells was observed, possibly indicating a systemic increase in T cell activation (P = 0.03; Fig. 6 C). In contrast to the small increase in activation markers observed in unstimulated cells, when CD4+ T cells were isolated from M1-treated mice and stimulated ex vivo with anti-CD3/CD28 antibodies (in the absence of added inhibitors), these cells showed a hyperresponsive phenotype, producing substantially more IL-2 than cells isolated from control animals (P = 0.005; Fig. 6 D). These findings provide further evidence for a co-stimulatory effect of IAP antagonism and indicate that IAP antagonists may be able to augment immune responses in vivo.
Figure 6.
Systemic delivery of IAP antagonists is well tolerated and leads to T cell hyperresponsiveness. (A–D) Mice were administered 750 µg M1 daily for 1 wk by gastric lavage. Spleen cells were harvested and analyzed by flow cytometry using the indicated markers. Six animals were used per group. (A) Comparison of total CD4+ and CD8+ T cells in mice treated with M1 or control (Ctl.) compound. (B) Quantification of NK1.1/CD3 staining on spleen cells from M1-treated mice. (C, left) Flow cytometry plots showing CD69+CD3+ T cells in M1-treated and control animals. (right) Quantification of CD69/CD3 staining from the left panel. (D) 105 CD4+ T cells were isolated from mice treated with M1 and stimulated with anti-CD3/CD28 for 48 h. Labels refer to the treatment conditions of the mouse from which T cells were isolated for analysis; IL-2 was measured by ELISA. (A–D) Error bars represent SEM. Results are representative of two independent experiments.
IAP antagonists can enhance the potency of tumor vaccines
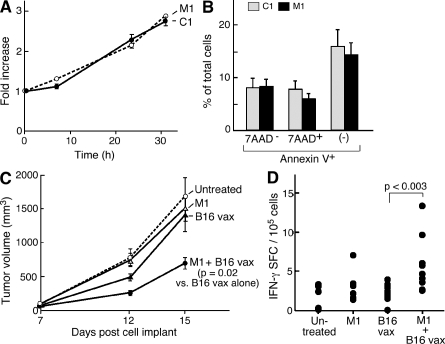
Based on these findings, we hypothesized that IAP antagonists could function to augment tumor vaccine–induced responses. To evaluate IAP antagonist activity in vivo, we first used a suboptimal tumor cell vaccine in which irradiated B16 mouse melanoma cells are used to protect against live B16 challenge in syngeneic C57BL/6 mice. This approach has low intrinsic potency, but vaccine efficacy can be enhanced through a variety of immune manipulations, making it a useful system for studying strategies for augmenting antitumor immunity (Dranoff et al., 1993; Jinushi et al., 2007). This system is further appropriate because we found that the IAP antagonists do not appear to have direct effects on B16 cells in culture. IAP antagonist treatment does not alter B16 proliferation in vitro (Fig. 7 A); furthermore, IAP antagonists do not enhance apoptosis or caspase cleavage in B16 cells after irradiation (Fig. 7 B and Fig. S7).
Figure 7.
IAP antagonists augment a prophylactic antitumor vaccine. (A) 104 B16 cells were plated in the presence of IAP antagonists or control compound. Fold increase was determined using CellTiter-Glo relative to time 0. (B) B16 cells were irradiated with 3.5 krad and cultured for 24 h in the presence of IAP antagonists or control compound. Apoptosis was assessed by flow cytometry using annexin V and 7AAD staining. Results are representative of at least two independent experiments. (C) Tumor growth curves for C57BL/6 mice injected with 5 × 105 B16 melanoma cells on day 0. Mice either did not receive a vaccine or received vaccines comprising 150 mg/kg M1, 5 × 105 irradiated B16 cells, or 150 mg/kg M1 and irradiated B16 cells before tumor challenge. Eight mice were used per group. Data are consistent with two independent experiments using several distinct schedules and doses. (A–C) Error bars represent SEM. (D) On day 14, 2.5 × 105 CD8+ T cells were isolated from the spleens of mice vaccinated as indicated and stimulated by irradiated spleen cells pulsed with TRP-2 peptide or vehicle. IFN-γ–reactive cells were determined by the enzyme-linked immunosorbent spot.
Consistent with the effect of IAP antagonists in vitro and previous experiments studying B16 vaccines (Dranoff et al., 1993), mice treated with either the IAP antagonist M1 or irradiated B16 melanoma cells alone showed no delay in tumor growth compared with controls after B16 challenge. However, combination treatment of mice with M1 and irradiated B16 cells led to a 65% reduction in B16 growth after challenge (P = 0.02; Fig. 7 C). This reduction was associated with a significant increase in the frequency of CD8+ T cells specific for the B16 antigen TRP-2 (Jinushi et al., 2007) in the spleens of mice treated with the combination therapy compared with animals treated with irradiated B16 alone, suggesting that, in part, the efficacy of the combined vaccine is mediated by an increase in T cell immunity (P = 0.003; Fig. 7 D; Jinushi et al., 2007). These findings establish as proof of principle that IAP antagonists can function as immunostimulants.
We next sought to assess the ability of IAP antagonists to augment immune responses in the context of a more efficacious tumor vaccine. Irradiated B16 vaccines comprised of cells engineered to secrete GM-CSF (GVAX) can provide potent antitumor immunity, completely protecting mice from subsequent tumor challenge (Dranoff et al., 1993, Jinushi et al., 2007). However, when used on established tumors in a therapeutic setting, GVAX slows tumor growth but does not result in tumor eradication, making this an appropriate model to study IAP antagonists in the setting of a potent but incompletely effective vaccine (Jinushi et al., 2007).
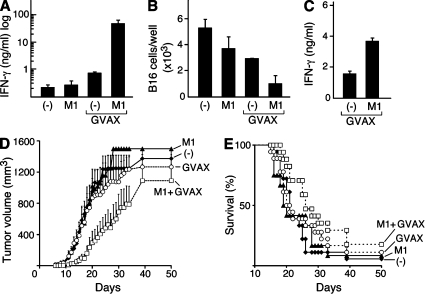
We first examined B16 responses in mice vaccinated with either GVAX or GVAX in combination with M1. In combination-treated mice, IAP antagonist treatment was begun on day 1 and continued until day 6. On day 7, the lymph nodes that drain the vaccine site were harvested and cultured with irradiated B16 cells. Lymph node cell cultures from mice treated with combination therapy produced substantially more IFN-γ in response to B16 cells than did cells taken from mice treated with either M1 or GVAX alone (P = 0.03; Fig. 8 A). Furthermore, significantly fewer B16 cells remained in the lymph node cultures derived from combination-treated mice compared with cultures from GVAX- or M1-treated animals (P = 0.03; Fig. 8 B). This loss of B16 cells was likely caused by enhanced cytotoxicity by cells from combination-treated animals because these cultures showed enhanced B16 cell killing in chromium release assays (unpublished data). In addition to lymph node responses, IFN-γ production from NK cells harvested from the spleens of vaccinated mice and co-cultured with YAC-1 cells was also enhanced in combination-treated animals compared with mice treated with GVAX alone (P < 0.0001; Fig. 8 C). Lymph nodes harvested from combination-treated animals were only marginally larger than lymph nodes from GVAX-treated mice, though, and contained similar numbers of B cells, T cells, and DCs, as well as activated and regulatory T cell subsets (Fig. S8). IFN-γ production was higher in lymph node cultures from combination-treated mice even when the differences in cell number were taken into account (unpublished data).
Figure 8.
IAP antagonists enhance immune responses to a therapeutic antitumor vaccine. (A–C) On day 0, mice were vaccinated with irradiated B16 cells engineered to secrete GM-CSF (GVAX) and were either left untreated or were given a 6-d course of 1,000 µg/day M1 by gastric lavage (GVAX M1). Untreated mice (−) and mice given M1 in the absence of GVAX (M1) were used as controls. (A and B) Vaccination site draining lymph nodes were harvested on day 7 and resuspended in 500 µl RPMI containing 10 U/ml recombinant human IL-2. Lymph node cells were incubated with 2 × 105 irradiated B16 cells for 4 d. (A) IFN-γ was measured in the culture supernatants by ELISA. (B) Total viable B16 cells were quantified by trypan blue exclusion. (C) 2 wk after vaccination, NK cells were isolated, activated, and analyzed as in Fig. 2 F. (A–C) Results are representative of at least two independent experiments with three to four mice per group. (D and E) On day 0, mice were challenged with 2 × 105 B16 cells. Mice were vaccinated as in A–C starting on day 1. Tumor growth (D) and survival (E) for mice after challenge are shown. Results represent the combination of three similarly designed, independent experiments with similar results for a total of 12–18 mice per group. (A–E) Error bars represent SEM.
We next examined whether IAP antagonist treatment could augment the effect of GVAX on tumor growth. Mice were vaccinated 1 d after B16 challenge with either GVAX alone or GVAX followed by daily treatmrnt with M1 for 1 wk. Combination-treated mice showed significantly decreased tumor growth rates compared with animals treated with GVAX or M1 alone (P < 0.05 from day 10–25), although median survival was not prolonged (Fig. 8, D and E). Although additional studies to identify optimal dosing regimens are required, these results confirm the immunomodulatory activity of the IAP antagonists and establish the ability of these drugs to improve responses to tumor vaccines in both prophylactic and therapeutic settings.
DISCUSSION
Collectively, our findings establish the IAPs as important regulators of T cell activation and define IAP antagonists as a novel class of immunomodulating agent. Unlike conventional adjuvants, IAP antagonists act in a co-stimulatory capacity, enhancing immune responses to physiological immune signals in both mice and humans, while lacking intrinsic stimulatory capacity. The co-stimulatory effect is striking in T cells where IAP antagonism in the context of stimulation leads to enhanced cytokine secretion, as well as increased proliferation and expression of activation markers.
IAP antagonism appears to co-stimulate T cells by blocking the ability of the cIAPs to inhibit alternative NF-κB signaling, likely through their ability to regulate NIK, as has been shown in a variety of other cell types (Gaither et al., 2007; Lu et al., 2007; Petersen et al., 2007; Varfolomeev et al., 2007, 2008; Vince et al., 2007, 2008; Bertrand et al., 2008; Mahoney et al., 2008; Vallabhapurapu et al., 2008; Zarnegar et al., 2008). T cells from mice harboring spontaneous mutations in NIK are resistant to IAP antagonism, and decreases in cIAP-1 and cIAP-2 after antagonist treatment are associated with rapid activation of alternative NF-κB. Similarly, knockdown of cIAP-1 and cIAP-2 enhances cytokine production from stimulated T cells.
In human T cells, cIAP-2 is regulated after signaling downstream of GITR. This finding indicates a potential physiological role for the IAPs in the regulation of GITR co-stimulation, which is known to depend in part on alternative NF-κB (Watts, 2005). Given the range of TNF family receptors now known to signal through the IAPs, this finding further suggests that in T cells, the IAPs may play a role downstream of TNF receptor co-stimulation more broadly. If this hypothesis is born out, then IAP antagonists could function as activators of multiple co-stimulatory receptors simultaneously, while bypassing any requirement for co-stimulatory receptor ligation. Such co-stimulatory activity by a small molecule may be useful in a wide variety of immunotherapies, including vaccine development and the treatment of immunodeficiencies. Indeed, several different agonistic mAbs against TNF family receptors are currently under investigation as targets for immunotherapy for cancer; these include antibodies to GITR, CD134 (OX40), and CD137 (4-1BB), with anti-CD137 antibodies now in phase I/II testing (Dougan and Dranoff, 2009).
In mice, we have shown that in vivo administration of IAP antagonists can augment the efficacy of both prophylactic and therapeutic tumor vaccines. IAP antagonist treatment is associated with systemic hyperresponsive T cells in the absence of overt autoimmunity or expansion of the T cell compartment. After vaccination, IAP antagonist treatment leads to augmented antitumor responses that are associated with delayed tumor growth and prolonged survival. These findings demonstrate that the effects of IAP antagonism observed in culture can, at least in part, be harnessed in vivo to modulate immune responses. Future work will be necessary to fully optimize this approach, in particular focusing on alternative conditions for IAP antagonist delivery and the introduction of optimal antigenic signals. Our results in cell culture suggest that certain threshold levels of stimulation substantially increase the efficacy of IAP antagonists, as indicated by the change in the magnitude of the IAP antagonist effect during anti-CD3 dose titration (Figs. 1 C and 2 A). Moreover, although we have not found evidence for apoptosis regulation by the IAPs in cell types examined in these experiments, further investigations may well reveal circumstances under which the antiapoptotic activity of the IAPs is indispensable for T cell survival or function.
The unanticipated activity of the IAP antagonists in T cells and other effectors of antitumor immunity also enables a novel approach to chemotherapy for cancer. IAP antagonists could have a synergistic effect on tumors. Through direct cytotoxicity, IAP antagonists could increase tumor cell death, leading to increased presentation of tumor antigens to the immune system. At the same time, we have now demonstrated that inhibiting IAPs removes a physiological signaling brake, allowing for enhanced responses from both CD4+ and CD8+ T cells as well as other key antitumor effector cells, including NKT cells and NK cells.
MATERIALS AND METHODS
Animals.
C57BL/6 and BALB/c wild-type mice were purchased from Taconic or The Jackson Laboratory or bred in house. Aly/aly mice were obtained from G. Benichou (Massachusetts General Hospital, Boston, MA; Miyawaki et al., 1994). FOXP3-GFP knockin mice were obtained from A. Rudensky (Memorial Sloan-Kettering Cancer Center, New York, NY; Fontenot et al., 2005). All animal experimentation was performed in accordance with institutional guidelines and the review board of Harvard Medical School, which granted permission for this study, and was approved by the Association for Assessment and Accreditation of Laboratory Animal Care–accredited Dana-Farber Cancer Institute Institutional Animal Care and Use Committee.
Antibodies.
All antibodies used for flow cytometry were obtained from BD. BCL10 (rabbit pAb; Cell Signaling Technology), caspase 3 (rabbit pAb; Cell Signaling Technology), human CD3 (mouse mAb clone HIT3a; BD), mouse CD3 (hamster mAb clone 145-2C11; BD), human CD28 (mouse mAb clone CD28.2; BD), mouse CD28 (hamster mAb clone 37.15; BD), cIAP-1 (goat pAb; R&D Systems), cIAP-2 (mouse mAb clone 315304; R&D Systems), GAPDH (mouse mAb clone MAB374; Millipore), GITR (mouse mAb DT5D3; Miltenyi Biotec), IκB-α (mouse mAb clone 112B2; Cell Signaling Technology), p100/p52 (rabbit pAb; Cell Signaling Technology), RelB (rabbit pAb; Cell Signaling Technology), USF-2 (rabbit pAb; Santa Cruz Biotechnology, Inc.), XIAP (clone 28; BD), and ZAP-70 (rabbit mAb clone D1C10E; Cell Signaling Technology) were also used.
Flow cytometry and immunoblotting.
Single cell suspensions were made from resected spleens or lymph nodes by mechanical disruption; red blood cells were removed by hypotonic lysis. CD4, CD8, or DX5+ cells were purified either from organ suspensions (mouse) or peripheral blood (human) using magnetic beads conjugated to the indicated antibodies (Miltenyi Biotec); purifications were performed according to manufacturer’s instructions. Cells were then washed and stained for 30 min on ice with the indicated antibodies in PBS with 1% inactivated fetal calf serum; for most experiments, Fc receptors were blocked using an unconjugated Fc-blocking antibody (BD) 15 min before staining. For Western blot analysis, cells were lysed in 1% NP-40 or using a cytoplasmic/nuclear fractionation kit (Thermo Fisher Scientific). Protein concentration was quantified by bicinchoninic acid assay (Thermo Fisher Scientific), and 30 µg of protein was loaded per lane onto 12% acrylamide gels. SDS-PAGE was followed by transfer to nitrocellulose and immunoblotting using the indicated primary antibodies. Secondary antibodies conjugated to alkaline phosphatase were purchased from Jackson ImmunoResearch Laboratories, Inc. and used at 1:5,000 dilution.
ELISA.
All ELISA kits were purchased from BD and used according to the manufacturer’s instructions.
Lentiviral knockdown.
2 × 104 CD4+ T cells isolated by positive selection from human peripheral blood were infected by spin transduction with highly concentrated lentiviral particles (Broad Institute and Sigma-Aldrich) using a multiplicity of infection of 3. 2 µg/ml puromycin was added 48 h after infection. Cells were analyzed 4–6 d later. Infections and subsequent cultures were performed in the presence of 2 µg/ml plate-bound anti-CD3, 2 µg/ml anti-CD28, and 20 U/ml recombinant human IL-2 in 100 µl RPMI. cIAP-1 KD1, 5′-GCCGAATTGTCTTTGGTGCTTCTCGAGAAGCACCAAAGACAATTCGGC-3′; cIAP-1 KD2, 5′-GCTGCGGCCAACATCTTCAAACTCGAGTTTGAAGATGTTGGCCGCAGC-3′; cIAP-1 KD3, 5′-TGGTTAAATCTGCCTTGGAAACTCGAGTTTCCAAGGCAGATTTAACCA-3′; cIAP-2 KD1, 5′-CAGTTCGTACATTTCTTTCATCTCGAGATGAAAGAAATGTACGAACTG-3′; cIAP-2 KD2, 5′-GCAGAGTCATCAATTATCCATCTCGAGATGGATAATTGATGACTCTGC-3′; and cIAP-2 KD3, 5′-GCACTACAAACACAATATTCACTCGAGTGAATATTGTGTTTGTAGTGC-3′ were used.
Anti-GITR co-stimulation.
3 × 106 CD4+ human T cells isolated from the peripheral blood by positive selection were incubated with either 2 or 10 µg/ml of plate-bound anti-CD3 and 2 µg/ml anti-CD28 for 24 h in 500 µl RPMI. After 24 h, T cells were either continued on anti-CD3/CD28 stimulation for an additional 14–24 h or stimulated with plate-bound anti-CD3/CD28 and 5 µg/ml anti-GITR for the same period of time. Co-stimulation was verified by measuring cytokine production in the culture supernatants by ELISA.
Ex vivo stimulation.
Mice were treated daily with M1 by gastric lavage at a dose of 750 µg per day. After 1 wk, mice were euthanized, and CD4+ T cells were isolated by positive selection using magnetic beads. 106 CD4+ T cells were incubated with 5 µg/ml of plate-bound anti-CD3 and 2 µg/ml anti-CD28 for 48 h in 500 µl RPMI.
Prophylactic B16 vaccine.
On days 0 and 7, mice were given a subcutaneous injection containing 5 × 105 irradiated (3.5 krad) B16 cells in conjunction with oral administration of M1 or control compound at either 150 or 30 mg/kg. On day 14, mice were challenged with a subcutaneous injection containing 5 × 105 live B16 cells.
B16 GVAX.
On day 0, mice were challenged with a subcutaneous injection containing 2 × 105 live B16 cells. On day 1, mice received a subcutaneous injection containing 5 × 105 irradiated (3.5 krad) B16 cells engineered to secrete GM-CSF (GVAX). On days 2–6, mice were given M1 at 1,000 µg per day by gastric lavage.
Enzyme-linked immunosorbent spot.
2.5 × 105 CD8+ T cells isolated by positive selection from vaccinated mouse spleens were incubated with 105 irradiated syngeneic mouse splenocytes pulsed with TRP-2 peptide (180–188: SVYDFFVWL) or vehicle control (DMSO) for 24 h in 100 µl RPMI media.
Ex vivo cultures.
Cells were isolated from the vaccine site draining lymph node or from the spleens of vaccinated mice by mechanical disruption; red blood cells were removed by hypotonic lysis. For B16 cultures, cells were incubated and analyzed as described in the figure legend. NK cells were isolated by DX5+ cell purification using magnetic beads (Miltenyi Biotec) and cultured as described in the figure legend.
Statistics.
Two sample comparisons used the t test with pooled variance if there was no evidence of inhomogeneity of variances between groups. If the variances were unequal, the exact Wilcoxon rank sum test, a nonparametric alternative to the t test, was used. Every effort was made to keep testing consistent across related experiments. For comparisons of more than two groups, analysis of variance was used if there was no evidence of inhomogeneity of variance; the Kruskal-Wallis test was the nonparametric alternative. Tumor growth experiments were analyzed using mixed model analysis of variance and the Welch’s t test.
Online supplemental material.
Fig. S1 shows relative cell counts and IL-2 production from IAP antagonist–treated CD4+ T cells stimulated with anti-CD3 at several concentrations as indicated. Fig. S2 shows IL-2 production from CD4+ T cells stimulated with anti-CD3/CD28 and treated with one of three distinct IAP antagonists, a control compound of similar structure, or vehicle. Fig. S3 shows cytokine production from IAP antagonist–treated OTI T cells stimulated with the OTI-specific agonist peptide SIINFEKL or whole OVA protein presented by formalin-fixed DCs at the indicated concentrations. Fig. S4 shows nuclear RelB and p52 levels in unstimulated, purified CD4+ T cells exposed to IAP antagonist or control compound. Fig. S5 shows the strategy used to purify naive T cells from +/aly and aly/aly spleens and confirms the purity of the post-sort populations. Fig. S6 shows cIAP-1 and cIAP-2 levels in unstimulated, purified, human CD4+ T cells exposed to IAP antagonist or control compound. Fig. S7 shows annexin V/7AAD (7-amino actinomycin D) staining and caspase 3 cleavage in cultured B16 cells exposed to IAP antagonists. Fig. S8 shows immune populations in the draining lymph node of mice vaccinated with GVAX or GVAX plus daily dosing of an IAP antagonist. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20101123/DC1.
Acknowledgments
We thank Gilles Benichou for providing aly/aly mice.
This work was supported by the Dana-Farber Cancer Institute/Novartis Program in Drug Discovery (grant to G. Dranoff) and grant F30AG030298 from the National Institute on Aging (to M. Dougan).
G. Dranoff is a grantee of and consultant to the Novartis Institute of Biomedical Research. J. Slisz, B. Firestone, D. Porter, and L. Zawel are employees of the Novartis Institute of Biomedical Research. The authors have no additional conflicts of interest.
Footnotes
Abbreviations used:
- GITR
- glucocorticoid-induced TNF receptor
- GM-CSF
- granulocyte-macrophage CSF
- IAP
- inhibitor of apoptosis protein
- NIK
- NF-κB–inducing kinase
- SEB
- staphylococcus enterotoxin B
- SMAC
- second mitochondrial-derived activator of caspases
- XIAP
- X-linked IAP
References
- Bauler L.D., Duckett C.S., O’Riordan M.X. 2008. XIAP regulates cytosol-specific innate immunity to Listeria infection. PLoS Pathog. 4:e1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand M.J., Milutinovic S., Dickson K.M., Ho W.C., Boudreault A., Durkin J., Gillard J.W., Jaquith J.B., Morris S.J., Barker P.A. 2008. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol. Cell. 30:689–700 10.1016/j.molcel.2008.05.014 [DOI] [PubMed] [Google Scholar]
- Bertrand M.J., Doiron K., Labbé K., Korneluk R.G., Barker P.A., Saleh M. 2009. Cellular inhibitors of apoptosis cIAP1 and cIAP2 are required for innate immunity signaling by the pattern recognition receptors NOD1 and NOD2. Immunity. 30:789–801 10.1016/j.immuni.2009.04.011 [DOI] [PubMed] [Google Scholar]
- Bidère N., Su H.C., Lenardo M.J. 2006. Genetic disorders of programmed cell death in the immune system. Annu. Rev. Immunol. 24:321–352 10.1146/annurev.immunol.24.021605.090513 [DOI] [PubMed] [Google Scholar]
- Conte D., Holcik M., Lefebvre C.A., Lacasse E., Picketts D.J., Wright K.E., Korneluk R.G. 2006. Inhibitor of apoptosis protein cIAP2 is essential for lipopolysaccharide-induced macrophage survival. Mol. Cell. Biol. 26:699–708 10.1128/MCB.26.2.699-708.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conze D.B., Albert L., Ferrick D.A., Goeddel D.V., Yeh W.C., Mak T., Ashwell J.D. 2005. Posttranscriptional downregulation of c-IAP2 by the ubiquitin protein ligase c-IAP1 in vivo. Mol. Cell. Biol. 25:3348–3356 10.1128/MCB.25.8.3348-3356.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csomos R.A., Wright C.W., Galbán S., Oetjen K.A., Duckett C.S. 2009. Two distinct signalling cascades target the NF-kappaB regulatory factor c-IAP1 for degradation. Biochem. J. 420:83–91 10.1042/BJ20082140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez E., Lee S.H., Gauthier S., Yaraghi Z., Tremblay M., Vidal S., Gros P. 2003. Birc1e is the gene within the Lgn1 locus associated with resistance to Legionella pneumophila. Nat. Genet. 33:55–60 10.1038/ng1065 [DOI] [PubMed] [Google Scholar]
- Dougan M., Dranoff G. 2009. Immune therapy for cancer. Annu. Rev. Immunol. 27:83–117 10.1146/annurev.immunol.021908.132544 [DOI] [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R.C. 1993. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc. Natl. Acad. Sci. USA. 90:3539–3543 10.1073/pnas.90.8.3539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot J.D., Rasmussen J.P., Williams L.M., Dooley J.L., Farr A.G., Rudensky A.Y. 2005. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 22:329–341 10.1016/j.immuni.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Gaither A., Porter D., Yao Y., Borawski J., Yang G., Donovan J., Sage D., Slisz J., Tran M., Straub C., et al. 2007. A Smac mimetic rescue screen reveals roles for inhibitor of apoptosis proteins in tumor necrosis factor-alpha signaling. Cancer Res. 67:11493–11498 10.1158/0008-5472.CAN-07-5173 [DOI] [PubMed] [Google Scholar]
- Greenwald R.J., Freeman G.J., Sharpe A.H. 2005. The B7 family revisited. Annu. Rev. Immunol. 23:515–548 10.1146/annurev.immunol.23.021704.115611 [DOI] [PubMed] [Google Scholar]
- Heemels M.T., Ploegh H. 1995. Generation, translocation, and presentation of MHC class I-restricted peptides. Annu. Rev. Biochem. 64:463–491 10.1146/annurev.bi.64.070195.002335 [DOI] [PubMed] [Google Scholar]
- Hogquist K.A., Jameson S.C., Heath W.R., Howard J.L., Bevan M.J., Carbone F.R. 1994. T cell receptor antagonist peptides induce positive selection. Cell. 76:17–27 10.1016/0092-8674(94)90169-4 [DOI] [PubMed] [Google Scholar]
- Hu S., Du M.Q., Park S.M., Alcivar A., Qu L., Gupta S., Tang J., Baens M., Ye H., Lee T.H., et al. 2006. cIAP2 is a ubiquitin protein ligase for BCL10 and is dysregulated in mucosa-associated lymphoid tissue lymphomas. J. Clin. Invest. 116:174–181 10.1172/JCI25641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru N., Kishimoto H., Hayashi Y., Sprent J. 2006. Regulation of naive T cell function by the NF-kappaB2 pathway. Nat. Immunol. 7:763–772 10.1038/ni1351 [DOI] [PubMed] [Google Scholar]
- Jinushi M., Nakazaki Y., Dougan M., Carrasco D.R., Mihm M., Dranoff G. 2007. MFG-E8-mediated uptake of apoptotic cells by APCs links the pro- and antiinflammatory activities of GM-CSF. J. Clin. Invest. 117:1902–1913 10.1172/JCI30966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P.J., Grabow S., Gray D., McKenzie M.D., Nachbur U., Huang D.C., Bouillet P., Thomas H.E., Borner C., Silke J., et al. 2009. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 460:1035–1039 10.1038/nature08229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Lin A. 2002. NF-kappaB at the crossroads of life and death. Nat. Immunol. 3:221–227 10.1038/ni0302-221 [DOI] [PubMed] [Google Scholar]
- Krieg A., Correa R.G., Garrison J.B., Le Negrate G., Welsh K., Huang Z., Knoefel W.T., Reed J.C. 2009. XIAP mediates NOD signaling via interaction with RIP2. Proc. Natl. Acad. Sci. USA. 106:14524–14529 10.1073/pnas.0907131106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leulier F., Lhocine N., Lemaitre B., Meier P. 2006. The Drosophila inhibitor of apoptosis protein DIAP2 functions in innate immunity and is essential to resist gram-negative bacterial infection. Mol. Cell. Biol. 26:7821–7831 10.1128/MCB.00548-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Lin S.C., Huang Y., Kang Y.J., Rich R., Lo Y.C., Myszka D., Han J., Wu H. 2007. XIAP induces NF-kappaB activation via the BIR1/TAB1 interaction and BIR1 dimerization. Mol. Cell. 26:689–702 10.1016/j.molcel.2007.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney D.J., Cheung H.H., Mrad R.L., Plenchette S., Simard C., Enwere E., Arora V., Mak T.W., Lacasse E.C., Waring J., Korneluk R.G. 2008. Both cIAP1 and cIAP2 regulate TNFalpha-mediated NF-kappaB activation. Proc. Natl. Acad. Sci. USA. 105:11778–11783 10.1073/pnas.0711122105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh R.A., Villanueva J., Kim M.O., Zhang K., Marmer D., Risma K.A., Jordan M.B., Bleesing J.J., Filipovich A.H. 2009. Patients with X-linked lymphoproliferative disease due to BIRC4 mutation have normal invariant natural killer T-cell populations. Clin. Immunol. 132:116–123 10.1016/j.clim.2009.03.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzawa A., Tseng P.H., Vallabhapurapu S., Luo J.L., Zhang W., Wang H., Vignali D.A., Gallagher E., Karin M. 2008. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 321:663–668 10.1126/science.1157340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki S., Nakamura Y., Suzuka H., Koba M., Yasumizu R., Ikehara S., Shibata Y. 1994. A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol. 24:429–434 10.1002/eji.1830240224 [DOI] [PubMed] [Google Scholar]
- Moffat J., Grueneberg D.A., Yang X., Kim S.Y., Kloepfer A.M., Hinkle G., Piqani B., Eisenhaure T.M., Luo B., Grenier J.K., et al. 2006. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell. 124:1283–1298 10.1016/j.cell.2006.01.040 [DOI] [PubMed] [Google Scholar]
- Petersen S.L., Wang L., Yalcin-Chin A., Li L., Peyton M., Minna J., Harran P., Wang X. 2007. Autocrine TNFalpha signaling renders human cancer cells susceptible to Smac-mimetic-induced apoptosis. Cancer Cell. 12:445–456 10.1016/j.ccr.2007.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D.H., Vance R.E., McMahon C.W. 2001. Regulation of the natural killer cell receptor repertoire. Annu. Rev. Immunol. 19:291–330 10.1146/annurev.immunol.19.1.291 [DOI] [PubMed] [Google Scholar]
- Rigaud S., Fondanèche M.C., Lambert N., Pasquier B., Mateo V., Soulas P., Galicier L., Le Deist F., Rieux-Laucat F., Revy P., et al. 2006. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 444:110–114 10.1038/nature05257 [DOI] [PubMed] [Google Scholar]
- Rumble J.M., Oetjen K.A., Stein P.L., Schwartzberg P.L., Moore B.B., Duckett C.S. 2009. Phenotypic differences between mice deficient in XIAP and SAP, two factors targeted in X-linked lymphoproliferative syndrome (XLP). Cell. Immunol. 259:82–89 10.1016/j.cellimm.2009.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schile A.J., García-Fernández M., Steller H. 2008. Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev. 22:2256–2266 10.1101/gad.1663108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkura R., Kitada K., Matsuda F., Tashiro K., Ikuta K., Suzuki M., Kogishi K., Serikawa T., Honjo T. 1999. Alymphoplasia is caused by a point mutation in the mouse gene encoding Nf-kappa b-inducing kinase. Nat. Genet. 22:74–77 10.1038/8780 [DOI] [PubMed] [Google Scholar]
- Srinivasula S.M., Ashwell J.D. 2008. IAPs: what’s in a name? Mol. Cell. 30:123–135 10.1016/j.molcel.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S., Matsuzawa A., Zhang W., Tseng P.H., Keats J.J., Wang H., Vignali D.A., Bergsagel P.L., Karin M. 2008. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat. Immunol. 9:1364–1370 10.1038/ni.1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varfolomeev E., Blankenship J.W., Wayson S.M., Fedorova A.V., Kayagaki N., Garg P., Zobel K., Dynek J.N., Elliott L.O., Wallweber H.J., et al. 2007. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNFalpha-dependent apoptosis. Cell. 131:669–681 10.1016/j.cell.2007.10.030 [DOI] [PubMed] [Google Scholar]
- Varfolomeev E., Goncharov T., Fedorova A.V., Dynek J.N., Zobel K., Deshayes K., Fairbrother W.J., Vucic D. 2008. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NF-kappaB activation. J. Biol. Chem. 283:24295–24299 10.1074/jbc.C800128200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vince J.E., Wong W.W., Khan N., Feltham R., Chau D., Ahmed A.U., Benetatos C.A., Chunduru S.K., Condon S.M., McKinlay M., et al. 2007. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 131:682–693 10.1016/j.cell.2007.10.037 [DOI] [PubMed] [Google Scholar]
- Vince J.E., Chau D., Callus B., Wong W.W., Hawkins C.J., Schneider P., McKinlay M., Benetatos C.A., Condon S.M., Chunduru S.K., et al. 2008. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1–TRAF2 complex to sensitize tumor cells to TNFα. J. Cell Biol. 182:171–184 10.1083/jcb.200801010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Du F., Wang X. 2008. TNF-alpha induces two distinct caspase-8 activation pathways. Cell. 133:693–703 10.1016/j.cell.2008.03.036 [DOI] [PubMed] [Google Scholar]
- Watts T.H. 2005. TNF/TNFR family members in costimulation of T cell responses. Annu. Rev. Immunol. 23:23–68 10.1146/annurev.immunol.23.021704.115839 [DOI] [PubMed] [Google Scholar]
- Xu L., Zhu J., Hu X., Zhu H., Kim H.T., LaBaer J., Goldberg A., Yuan J. 2007. c-IAP1 cooperates with Myc by acting as a ubiquitin ligase for Mad1. Mol. Cell. 28:914–922 10.1016/j.molcel.2007.10.027 [DOI] [PubMed] [Google Scholar]
- Yang Y.L., Li X.M. 2000. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 10:169–177 10.1038/sj.cr.7290046 [DOI] [PubMed] [Google Scholar]
- Zarnegar B.J., Wang Y., Mahoney D.J., Dempsey P.W., Cheung H.H., He J., Shiba T., Yang X., Yeh W.C., Mak T.W., et al. 2008. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nat. Immunol. 9:1371–1378 10.1038/ni.1676 [DOI] [PMC free article] [PubMed] [Google Scholar]