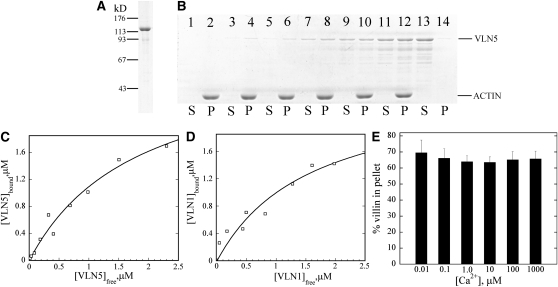
Figure 7.
VLN5 Binds to Actin Filaments with High Affinity.
(A) Recombinant VLN5 expressed in bacteria was purified to homogeneity with several chromatography steps. Three micrograms of purified protein separated on an SDS-PAGE gel and stained with Coomassie blue is shown.
(B) A high-speed cosedimentation assay was employed to determine the affinity of VLN5 for actin filaments. Three micromolar polymerized actin was incubated with various concentrations of VLN5 and the mixtures were centrifuged at 200,000g for 1 h to separate bound versus unbound VLN5. After centrifugation, equal amounts of pellets (P) and supernatants (S) were separated by SDS-PAGE and stained with Coomassie blue. Samples loaded in lanes 1 to 12 contained actin plus 0 (lanes 1 and 2), 0.1 (lanes 3 and 4), 0.2 (lanes 5 and 6), 0.5 (lanes 7 and 8), 1 (lanes 9 and 10), and 2 μM (lanes 11 and 12) VLN5, whereas lanes 13 and 14 contained 2 μM VLN5 only.
(C) Actin filaments were sedimented in the presence of various concentrations of VLN5, as shown in (B). The amount of VLN5 in pellets and supernatants from the various samples was determined with Image J. The concentration of VLN5 in the pellet ([VLN5]bound) as a function of the concentration of VLN5 in the supernatant ([VLN5]free) was plotted, and the data were fitted with a hyperbolic function to calculate the equilibrium dissociation constant (Kd) value. In this representative experiment, the calculated Kd value was 0.55 μM.
(D) The Kd value for VLN1, used as a positive control for binding experiments, was determined to be 0.63 μM in this representative experiment.
(E) To determine the effects of Ca2+ on VLN5 binding to actin filaments, 1 μM VLN5 was incubated with 3 μM F-actin in the presence of various [Ca2+]free. The percentage of VLN5 that sedimented with actin filaments was plotted as a function of [Ca2+] free. Error bars represent ±sd (n = 3).