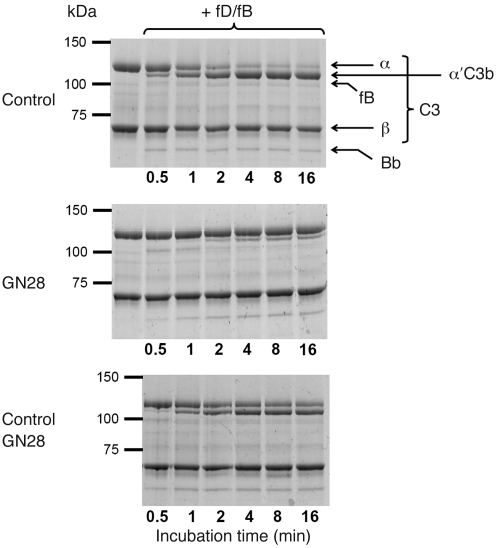
Figure 3. Total C3 from GN28 is only partially cleaved to C3b in the presence of fB and fD.
Coomassie-stained gels correspond to the SDS-PAGE analyses of C3 purified from GN28 and a normal control after incubation with fB and fD. The experiment was repeated twice with identical results. Top: C3 purified from normal individuals was rapidly and completely activated to C3b (determined by cleavage of the C3 α chain) in the presence of fB and fD. This activation correlated with consumption of fB and the appearance of the Bb fragment, indicating formation of the AP C3-convertase. Middle: Same experiment with C3 purified from GN28. Despite formation of the AP C3-convertase (demonstrated by consumption of fB and generation of the Bb fragment), only a small proportion of the C3 was activated to C3b. This suggests that C3 purified from the GN28 plasma contains 2 different C3 forms (WT and mutant protein), and that only C3WT is cleaved to C3b. Bottom: To rule out the presence of inhibitors in the C3 preparation from the GN28 plasma, a mixing experiment (equivalent amounts of control and GN28 C3) showed that addition of fB and fD caused activation of 50% of the C3, likely C3WT.