Figure 5.
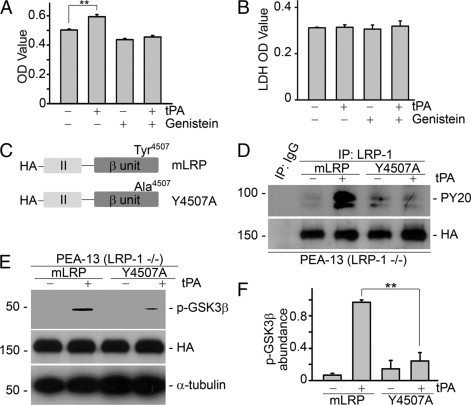
Phosphorylation of tyrosine residue Tyr4507 on LRP-1 is essential for tPA activated proliferative signaling and cell growth. A: Pretreatment with the specific tyrosine kinase inhibitor genistein (50 μmol/L) for 30 minutes abolished tPA-induced proliferation in NRK-49F fibroblasts. **P < 0.01, n = 4. B: No evident cytotoxicity of 50 μmol/L genistein treatment was detected by lactate dehydrogenase (LDH)-cytotoxicity assay (units are arbitrary). C: Graphic illustration of the structure of LRP-1 minireceptor (mLRP) and its mutant. Tyrosine residue Tyr4507 was mutated to alanine (Y4507A) by site-directed mutagenesis. D: tPA failed to induce tyrosine phosphorylation of LRP-1 in PEA-13 (LRP-1−/−) cells transfected with mutant Y4507A plasmid. PEA-13 fibroblasts were transfected with mLRP and mutant Y4507A plasmids followed by treatment with vehicle or tPA (10 nmol/L) for 0.5 minutes. Cell lysates were then immunoprecipitated by mouse IgG or mouse anti–LRP-1 antibody followed by immunoblotting with specific anti-tyrosine phosphorylation antibody PY20 and anti-HA antibody. E and F: Ectopic expression of mutant Y4507A plasmid abolished tPA-induced GSK3β phosphorylation. PEA-13 fibroblasts were transfected with mLRP and mutant Y4507A plasmids followed by treatment with vehicle or tPA (10 nmol/L) for 1 hour. Cell lysates were subjected to Western blot with anti–phospho-GSK3β, anti-HA, and anti–α-tubulin antibodies. Representative Western blot (E) and graphic demonstration of the relative abundance of phospho-GSK3β (F). **P < 0.01, n = 3.
