Figure 6.
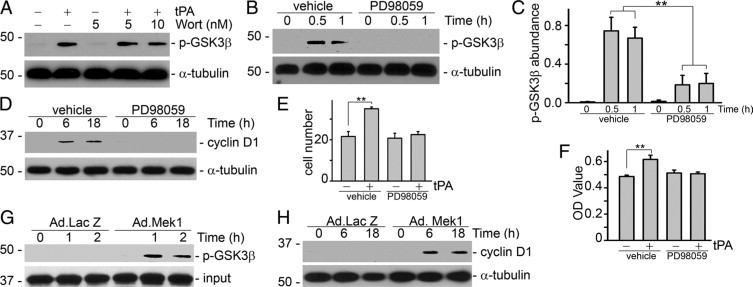
Erk1/2 activation is necessary and sufficient for the mitogenic effect of tPA. NRK-49F cells were pretreated with PI3K inhibitor wortmannin (Wort, 5 and 10 nmol/L) or Mek1 inhibitor PD98059 (20 μmol/L) for 30 minutes and then incubated with tPA (10 nmol/L) for various time periods as indicated. Cell lysates were probed with phospho-GSK3β, cyclin D1, and α-tubulin. A: Pretreatment with the PI3K inhibitor did not affect tPA-induced GSK3β phosphorylation. B and C: PD98059 abrogated tPA-induced GSK3β phosphorylation. B: Representative Western blot. C: Quantitative representation of the relative abundance of phospho-GSK3β. **P < 0.01, n = 3. PD98059 also suppressed tPA-induced cyclin D1 expression (D) and cell proliferation (E and F). Cell growth was evaluated by cell number count (E, ×104) and WST-8 assay (F). **P < 0.01, n = 4–6. Infection of constitutively active Mek1 adenovirus (Ad.MEK1) was sufficient to induce GSK3β phosphorylation (G) and cyclin D1 expression (H). NRK-49F cells were infected with adenovirus for periods of time as indicated. An adenoviral vector containing the β-galactosidase gene (Ad. LacZ) was used as a control.
