Abstract
Background
We have previously shown that the rate of habituation of the heart rate orienting response to a novel odor in rats is negatively affected by neonatal ethanol exposure. Thus far, however, only young rats (16 days of age) have been tested. Given the persistence of attention and memory problems evident in humans exposed to ethanol in utero, the purpose of this experiment was to examine the longer-term consequences of ethanol exposure on response habituation.
Methods
Ethanol (5.25g/kg/day) was administered intragastrically to male and female Sprague-Dawley rats on postnatal days (PD) 4-9, and controls were given sham intubations. Animals were tested for heart rate orienting and response habituation to a novel olfactory stimulus (amyl acetate) on PD 16, 23, or 30.
Results
Animals tested on PD 16 or 23 showed normal heart rate deceleration to the novel odor, a measure of the orienting response. However, ethanol-treated subjects showed impaired response habituation compared to sham controls. While controls exhibited complete habituation within 4–5 trials, ethanol-treated animals continued to respond throughout the testing session, with little decrement in heart rate response magnitude across 10 stimulus presentations. A different pattern of responding was observed in animals tested during adolescence (PD 30). Control animals failed to show the typical heart rate decrease indicative of orienting, and instead showed a tendency toward tachycardia. In contrast, ethanol-treated animals tested on PD 30 showed orienting bradycardia that persisted for several trials.
Conclusions
These data suggest that there are relatively long-term consequences of neonatal ethanol exposure on nonassociative memory. This impairment in habituation may be relevant to the distractibility and poor focused attention that is pervasive among humans diagnosed with fetal alcohol spectrum disorders.
Keywords: fetal alcohol spectrum disorders, nonassociative learning, alcohol, olfactory, rat
Cognitive outcomes associated with prenatal exposure to alcohol include low IQ, impaired short-term memory, problems with executive function, poor spatial abilities, and attention deficits. Longitudinal studies in humans prenatally exposed to alcohol have documented the persistence of such effects. Early exposure to alcohol can lead to developmental delays or more permanent impairments in neurocognitive functioning (Connor et al., 1999; Kerns et al., 1997; Korkman et al., 2003; Riley, 1990). Studies have found that although the physical abnormalities associated with prenatal alcohol exposure may normalize with maturation, impairments in attention, memory and executive function can persist into adulthood (Connor and Streissguth, 1999; Kodituwakku, 2007; Streissguth, 2007).
Disturbances in attentional processes especially have been consistently reported across the lifespan of individuals with known prenatal exposure to alcohol. Such disruptions in attention have been noted as early as the first day of life (Streissguth et al., 1983), throughout childhood (Coles et al., 1997; Nanson and Hiscock, 1990; Streissguth et al., 1984), during adolescence (Brown et al., 1991; Coles et al., 2002; Korkman et al., 2003), and into adulthood (Connor et al., 1999; Kerns et al., 1997; Streissguth, 2007). Attentional problems are often noted as decreased focus, poor sustained attention and vigilance, and distractibility. Kerns et al. (1997) reported that adults with a diagnosis of fetal alcohol syndrome (FAS) were more distractible during a vigilance task. Distractibility may in part result from deficits in habituation to sensory stimuli outside the realm of the task, and such disturbances have often been described as a lack of ability to tune-out redundant and task-irrelevant information (Connor and Streissguth, 1999; Streissguth et al., 1989).
Measurements of stimulus orienting have been powerful tools for examining attention, perception, learning and memory. The orienting response (OR) consists of a collection of central, autonomic, and behavioral reactions to the detection of a novel stimulus (e.g. Graham and Clifton, 1966; Sokolov, 1963). The most commonly used indices of orienting include behavioral orientation toward the source of stimulation and autonomic responses, such as changes in heart rate (Campbell et al., 1992; Lang et al., 1997). The heart rate component of the OR is defined as a decrease in rate (bradycardia), mediated primarily by activation of the parasympathetic nervous system (Graham, 1992; Hunt et al., 1994). Parasympathetic activation is functional in that it serves to reduce background noise, redistribute blood flow away from skeletal muscles to brain areas required for attention and vigilance, and can support faster reaction times (Lacey and Lacey, 1970). A fundamental property of the OR is its ability to undergo rapid habituation, which makes the OR suitable to the study of stimulus encoding and both short- and long-term recognition memory processes (e.g. Richardson and Campbell, 1991).
The rate of response habituation has been considered by many as an index of cognitive development in humans and other animals, and rate of habituation assessed in infancy has been regarded as a predictor of later intelligence (e.g. Bornstein, 1989; Kavšek, 2004; McCall and Carriger, 1993). According to comparator theories (e.g. Sokolov, 1963), habituation of the orienting response reflects short-term nonassociative memory processes, as it involves the recognition that a stimulus has been encountered previously and is not biologically significant. Infant recognition memory performance also predicts later IQ (Fagan and Singer, 1983; Rose and Feldman, 1995). Indeed, McCall (1994) has proposed that inhibition is a common feature in habituation and recognition memory paradigms and that it is the propensity for inhibition that accounts for the predictive relationship between these measures and later intelligence test scores. Thus, habituation may be useful in identifying alcohol-induced cognitive deficits soon after birth that could be predictive of more complex and persistent problems that span at least into adolescence and early adulthood (Carmichael Olson et al., 1998; Kerns et al., 1997).
Orienting responses have been examined in infants with a history of prenatal alcohol exposure. Coles et al. (1987) for example, reported that moderate gestational exposure to ethanol was correlated with reduced orienting and altered motor development in infants less than one month of age. Furthermore, these researchers found that the initial differences in orienting and motor competence were highly predictive of mental and motor performance assessed at 6 months. Streissguth et al. (1983) reported that infants exposed to moderate doses of ethanol during gestation exhibited reduced rates of response habituation to both auditory and visual stimuli when tested as early as 24 h after birth, with no obvious differences in initial orienting. This same cohort has been assessed repeatedly and deficits in attention and memory domains have consistently been reported (for review see Streissguth, 2007).
Previous studies from our laboratory, using young rats, support the findings of Streissguth et al. (1983) in that ethanol-treated animals exhibit impaired response habituation with no observable change in stimulus orienting. Specifically, rats given ethanol on postnatal days (PD) 4-9, to model third-trimester gestational exposure in humans (Dobbing and Sands, 1979; Goodlett and Johnson, 1999), exhibit deficits in habituation of the heart rate orienting response to a novel olfactory stimulus (Hunt and Morasch, 2004; Hunt and Phillips, 2004). However, in these experiments the subjects were tested at only one age, shortly after the termination of the ethanol administration period, on PD 16. Given the well-documented persistence of attention and memory impairments observed in humans with prenatal exposure to ethanol, the purpose of the present experiment was to examine whether ethanol-induced attenuation in rate of response habituation would persist beyond the period of infancy.
METHODS
Subjects
A total of 94 male and female Sprague-Dawley-derived rats representing 19 litters served as subjects in this experiment. Animals were born and reared in a temperature- and humidity-controlled vivarium in the Psychology Department at the College of William and Mary. Male and female breeders (Charles River Laboratories, Wilmington, MA) were housed together in 50.8 × 40.6 × 21.6 cm clear polycarbonate cages with wire lids and pine chip bedding. Animals had free access to high-protein rodent chow (LabDiet Formula 5008) and water. Cages were checked daily for the presence of pups, and the day of birth was designated as Postnatal Day 0 (PD 0). Litters were culled to 8–10 pups on PD 2. Pups remained housed with the dam and sire until PD 21, at which time they were weaned and group-housed with siblings in identical polycarbonate cages and in the same vivarium for the remainder of the experiment. The vivarium was maintained on a 14:10 h light:dark cycle with light onset at 0600 h. All procedures occurred during the light portion of the cycle and were approved by the College of William and Mary Institutional Animal Care and Use Committee.
Animals tested at 16 or 23 days of age were derived from the same 7 litters. The litter was equally divided between the two testing ages and no more than one male and one female pup from each neonatal treatment condition (ethanol or sham) was tested at a given age. Ethanol-and sham-treated animals tested at 30 days were derived from an additional 7 litters, and no more than one male and one female from each neonatal treatment group was tested. The remaining 30 day old animals from each litter were tested in a different behavioral paradigm, the results of which will be reported at a later date. Finally, a group of Unhandled control animals was tested at 30 days of age and subjects were from 5 additional litters. This group was included because of an unexpected finding in the 30-day-old sham-intubated control group. These animals were obtained from the same animal colony as the other subjects but were not treated during the neonatal period. No more than one male and one female pup per litter were tested at a given age, and approximately equal numbers of males and females were included in each group.
Apparatus
Heart rate was recorded using two transcutaneous electrodes made from 27 ga stainless steel wire and shaped to resemble small safety pins. The electrodes and attached teflon-coated lead wires (32 ga; Alpha Wire Co., Elizabeth, NJ) were acutely implanted prior to test. Cardiac potentials were amplified with a Grass Instruments (Quincy, MA) Model P15 preamplifier. The R-spike activated a Schmitt trigger (Coulbourn Instruments, Allentown, PA). A computer stored each inter-beat interval (IBI), measured to the nearest millisecond, and controlled all timing sequences and data collection. The cardiac signal was continuously displayed on an oscilloscope (Hitachi Model V-212).
Testing occurred in a 25-cm long cylindrical Plexiglas chamber (14 cm diameter) mounted horizontally inside a sound attenuating shell (66 × 37 × 81.5 cm). A 7.5-watt white light bulb was mounted on the inside of the shell to provide constant low-level illumination. The olfactory stimulus (0.5 ml amyl acetate + 40 ml water) was introduced into the chamber by means of a custom-made olfactometer system which has been described in detail elsewhere (Hunt et al., 1997b). The temperature inside the chamber was maintained at 28–32°C, depending on the age of the animals at test, by a heated airstream. The olfactory stimulus was evacuated from the chamber by negative pressure generated by an exhaust fan. Subjects were weighed using an Ohaus top-loading balance (Model GT 8000; Florham Park, NJ), accurate to .01g
Procedure
Ethanol Administration
On PD 4 animals were removed from the home cage. The litter was placed as a group in a 35.2 × 21.9 × 13.0 cm opaque polyethylene holding cage that was maintained at approximately 34°C by a heating pad placed beneath it. Four animals (two male and two female) were assigned to receive the ethanol treatment and four animals (two males and two females) were assigned to the sham control group. The ethanol-exposed subjects (EtOH) were administered 2.625 g/kg ethanol intragastrically twice per day, for a total daily dose of 5.25 g/kg. Intubations were achieved by using a 15-cm length of polyethylene tubing (PE-10, Clay-Adams, Sparks, MD) lubricated with corn oil and attached to a 1-ml syringe. The ethanol solution was 11.9% v/v 95% ethanol (Sigma Chemicals, St. Louis, MO) mixed with Similac® (Abbott Laboratories, Columbus, OH). The two ethanol administrations were separated by 2 h. Although blood-alcohol concentrations (BACs) were not measured in this experiment, data from other laboratories using this same dose have reported BACs in the range of 265–325 mg/dl (Goodlett & Johnson, 1997; Stanton & Goodlett, 1998). A third feeding of the Similac® vehicle was given 2 h after the second ethanol administration to supplement nutrition. Sham-intubated controls were subjected to the tube insertion procedure three times daily, but no fluid was delivered because administration of milk vehicle can cause abnormal weight gain (e.g. Goodlett and Johnson, 1997). The litter was immediately returned to the home cage after each of the three daily feedings. Following the final intubation on PD 9 pups were ear marked to denote neonatal treatment condition. On PD 21 all pups from the litter were weaned and group housed for the duration of the experiment. Unhandled control subjects that were tested at 30 days of age were left undisturbed, except for routine maintenance, during this time.
Heart Rate Testing
Separate groups of animals were tested on either PD 16, 23 or 30 (+/− 1 day). For test, heart rate electrodes were acutely implanted, one at the nape of the neck and the other 1 cm from the base of the tail. Electrode implantation required approximately 10 s per subject and was performed without anesthetic, as the procedure induces no more discomfort than a subcutaneous injection. In addition, electrodes and attached lead wires did not appear to interfere with general comfort or subject movement. Immediately after electrode implantation, subjects were placed into the test chamber for a 15 min period of adaptation (Saiers et al., 1990). Next, animals were given 10 presentations of the 10 s olfactory stimulus, separated by 100–200 s intervals. Inter-beat intervals (msec) were recorded for 1 s prior to each stimulus, during the 10 s of the stimulus, and for 5 s post-stimulus offset on each trial.
Treatment of Heart Rate Data
Inter-beat intervals were converted into a beats-per-minute (BPM) measure for analysis. Heart rate recorded during the 1 s baseline period was subtracted from that recorded during each second of the stimulus and post-stimulus periods to obtain difference scores. Negative difference scores reflected a decrease in heart rate (bradycardia) that defines the heart rate orienting response (Graham and Clifton, 1966), whereas positive difference scores reflected an increase in heart rate (tachycardia).
Statistical analyses
Heart rate data obtained from ethanol- and sham-treated subjects were analyzed using mixed-design Analysis of Variance (ANOVA), with age and neonatal treatment as between-groups variables and seconds (orienting response) or trial block (habituation) as the within-subjects variable. Sex was not included in the data analysis because of the small number of male and female subjects in each treatment group. In all cases involving a repeated measure, the Greenhouse-Geisser correction procedure was used to control for possible inflation of probability values (Keppel, 1982). The second-by-second changes from baseline heart rate obtained from the first test trial were analyzed separately to assess the integrity of the orienting response. Heart rate data from all trials were averaged across blocks of two trials for analysis of habituation. The peak change in heart rate on a given trial was defined as the largest change observed, either positive or negative, and has been used previously to describe this type of data (Hunt and Morasch, 2004; Hunt and Phillips, 2004). Data from sham-treated and unhandled control groups tested at 30 days of age were compared statistically using ANOVA. It should be noted that these two control groups did not differ on any of the measures, as reported below. Where appropriate, post hoc comparisons were made using Newman-Keuls tests (p < .05).
RESULTS
During the ethanol administration procedure (PD 4-9), 6 animals assigned to the EtOH group and 2 assigned to the Sham group were lost due to improper intubations. Heart rate data from an additional 15 animals were lost due to equipment malfunction or excessive noise in the heart rate signal. Data from the remaining 71 subjects were analyzed and the final ns per group are indicated in Table 1. There were approximately equal numbers of males and females in each of the groups.
Table 1.
Mean (+/− SEM) body weights (g) recorded from subjects during the ethanol administration period, postnatal days 4–9, and on the day of heart rate testing (postnatal day 16, 23 or 30). Final group sizes (ns) at test are indicated. Ethanol-treated subjects weighed less than sham controls beginning on postnatal day 5 and continuing throughout testing.
| Neonatal Treatment | Postnatal Day | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ethanol Administration | Heart Rate Testing | ||||||||
| 4 | 5 | 6 | 7 | 8 | 9 | 16 | 23 | 30 | |
| Ethanol | 14.4 (0.25) | 15.4 (0.28) | 17.4 (0.33) | 19.1 (0.39) | 20.9 (0.45) | 22.9 (0.53) | 37.4 (3.07) n = 9 |
62.9 (3.45) n = 10 |
103.5 (3.45) n = 8 |
| Sham | 14.0 (0.25) | 16.6 (0.28) | 19.1 (0.32) | 22.2 (0.38) | 24.8 (0.44) | 28.0 (0.52) | 41.9 (2.98) n = 12 |
70.5 (3.18) n = 11 |
110.9 (3.67) n = 11 |
| Unhandled | 108.7 (4.18) n = 10 |
||||||||
Body Weights
Body weights of animals recorded during the ethanol administration procedure (PD 4-9) and on the day of testing are shown in Table 1. Body weights recorded on PD 4-9 were analyzed using a 2 (neonatal treatment) × 6 (day) mixed-design ANOVA. This analysis revealed main effects of neonatal treatment and day, and a Neonatal Treatment x Day interaction [smallest F (1, 74) = 25.40, p < .001]. Post hoc Newman-Keuls tests indicated that on PD 4 animals had similar body weights, but beginning on PD 5 the ethanol-exposed animals weighed less than the sham controls. Both groups gained weight during the procedure, but the ethanol-exposed animals gained less than the sham controls.
Body weights recorded on the day of heart rate testing were analyzed using a 3 (age) × 2 (neonatal treatment) between-groups ANOVA. The analysis yielded main effects of age [F (2, 67) = 246.98, p < .001] and neonatal treatment [F (1, 67) = 5.02, p < .05]. Post hoc comparisons indicated that body weights increased with age and that ethanol-treated subjects weighed less than sham controls. Additionally, body weights of 30-day-old sham-treated and unhandled animals did not differ [F < 1].
Baseline Heart Rate
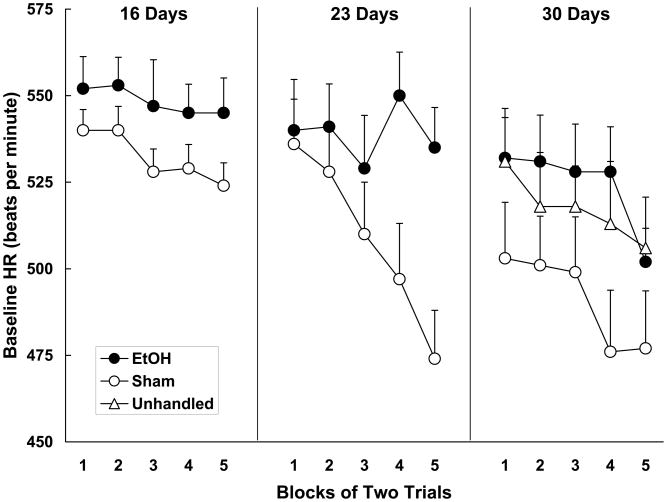
Baseline heart rates recorded during the test session are depicted in Figure 1. Baseline heart rates were analyzed using a 3 (age) × 2 (neonatal treatment) × 5 (trial block) mixed-design ANOVA. This analysis yielded significant main effects of age, neonatal treatment and blocks, as well as Neonatal Treatment x Blocks and Age x Neonatal Treatment x Blocks interactions [smallest F (4, 220) = 3.42, p < .05]. Follow-up ANOVAs (neonatal treatment x blocks) were conducted on the data obtained from each testing age. The ANOVAs yielded main effects of block at each age [smallest F (4, 76) = 2.85, p < .05]. The ANOVA conducted on the data from the 23 days old subjects additionally yielded a Neonatal Treatment x Block interaction, F (4, 76) = 5.29, p < .01. Generally, baseline heart rates of all subjects declined across the test session. For 23 day olds, however, the sham control group exhibited a more robust decline in baseline heart rate than the ethanol-treated group. Finally, an ANOVA comparing baseline heart rates of sham-treated and unhandled subjects tested at 30 days of age yielded no significant differences between the two control groups [F < 1].
Figure 1.
Mean (+/− SEM) beats-per-minute baseline heart rate (HR) recorded during the test session in 16-, 23- and 30-day-old animals. Baseline heart rate was averaged across blocks of two trials. Data are from ethanol-treated and sham control animals tested at each age, and also from an unhandled group tested at 30 days. EtOH animals were administered 5.25 g/kg/day ethanol on postnatal days 4-9. Sham controls were also treated on days 4-9 but received no ethanol. Generally, baseline heart rates decreased across the test session and there was little evidence of differences between the groups. The exception was the 23-day-old subjects; the sham control group tested at this age showed a larger decline than the EtOH group.
Heart Rate Orienting Response
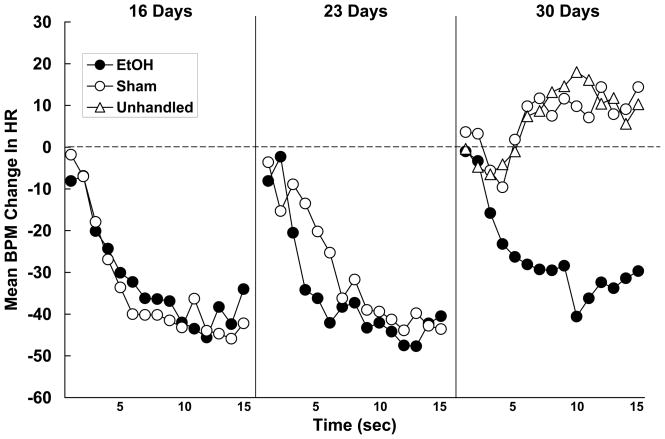
The heart rate responses of subjects tested at 16-, 23- or 30-days of age recorded on the first olfactory stimulus presentation are shown in Figure 2. Neonatal ethanol administration did not impact the heart rate orienting response in subjects tested at 16- or 23-days of age. The form and magnitude of the response observed in these subjects was nearly identical to that previously observed in experimentally-naïve subjects (e.g. Hunt and Phillips, 2004; Hunt et al., 1994; Hunt et al., 1997b; Sananes et al., 1988). In contrast however, ethanol had a dramatic effect on the heart rate response displayed by 30-day-old subjects. Thirty-day-old control animals failed to respond to the novel olfactory stimulus with heart rate deceleration whereas ethanol-treated animals did. The heart rate response exhibited by ethanol-exposed subjects was highly similar to that observed at the younger ages. These interpretations were confirmed statistically. The second-by-second changes in heart rate were analyzed using a 3 (age) x 2 (neonatal treatment) x 15 (seconds) mixed-design ANOVA. The analysis revealed significant main effects of age, neonatal treatment and seconds [smallest F (1, 55) = 6.35, p < .05]. The Age x Neonatal Treatment, Age x Seconds, and Age x Neonatal Treatment x Seconds interactions were also significant [smallest F (28, 770) = 2.05, p < .05]. The 3-way interaction was further explored with the use of contrast analyses (SAS v. 9.1; p < .05) to compare each group’s change in heart rate against its own baseline. These analyses confirmed that the interaction resulted from the 30-day-old Sham subjects failing to show a significant heart rate orienting response to the olfactory stimulus. In addition, all other groups responded on the first trial with substantial and roughly equivalent bradycardia. There was a tendency for the 30-day-old sham control animals to respond with an increase in heart rate (tachycardia), although this failed to reach statistical significance. The response of the 30-day-old Unhandled group, also shown in Figure 2, did not differ from that of the Sham group [F < 1]. That is, experimentally-naïve 30-day-olds also failed to exhibit a reliable heart rate orienting response to the olfactory stimulus.
Figure 2.
Mean second-by-second beats-per-minute (BPM) changes in heart rate (HR) recorded on the first presentation of the olfactory stimulus in animals tested at 16-, 23- or 30-days of age. EtOH animals were administered 5.25 g/kg/day ethanol on postnatal days 4-9, and these subjects were compared to animals not administered ethanol (sham or unhandled on postnatal days 4-9). The dotted line represents baseline heart rate, and decreases in heart rate (negative scores) define the orienting response. At 16 and 23 days, ethanol treatment had no effect on the OR. At 30 days the control groups failed to respond with orienting bradycardia while the EtOH group did.
Habituation of the Heart Rate Orienting Response
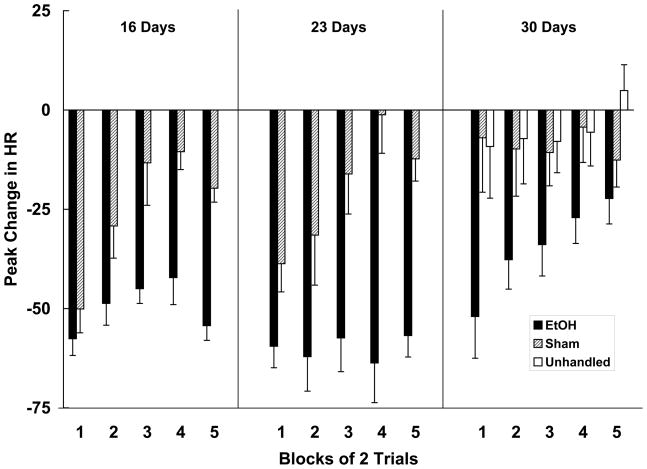
The peak changes in heart rate recorded on trial blocks 1–5 are shown in Figure 3. The data were analyzed using a 3 (age) x 2 (neonatal treatment) x 5 (trial block) mixed ANOVA. This analysis yielded main effects of age, neonatal treatment and trial block [smallest F (4, 220) = 6.62, p < .001]. The Age x Neonatal Treatment x Trial Block interaction was also significant, F (8, 220) = 2.38, p < .05. Post hoc Newman-Keuls tests were conducted to evaluate the three-way interaction. At 16- and 23-days of age the ethanol-treated subjects exhibited persistent heart rate orienting responses to the olfactory stimulus throughout the test session, with little evidence of response decrement. In contrast, control subjects at these ages exhibited response habituation that was evident by the third trial block. Rate of orienting response habituation in sham-treated subjects is comparable to that previously reported in subjects of about this age (e.g. Hunt et al., 1997a; Hunt and Phillips, 2004; Siegel et al., 1987). A different pattern was revealed at 30 days of age. At this age, control subjects showed little evidence of responding to the olfactory stimulus during the session, while ethanol-treated animals exhibited bradycardia on the first few trials. Ethanol-treated subjects did exhibit habituation, although the magnitude of the heart rate response of these subjects was greater than that of sham controls on all trial blocks except block 5. Subjects in the unhandled group, also shown in Figure 3, did not differ in their heart rate response to the olfactory stimulus from sham-treated controls [F < 1].
Figure 3.
Peak (Mean +/− SEM) beats-per-minute (BPM) changes in heart rate (HR) recorded during the 5 trial blocks of testing from 16-, 23- and 30-day old subjects. Changes in heart rate reflect the average of two trials. EtOH animals were administered 5.25 g/kg/day ethanol on postnatal days 4-9. Controls were either sham-intubated or unhandled on postnatal days 4-9. Rate of habituation was severely compromised in EtOH subjects tested at 16 and 23 days of age. For subjects tested at 30 days, the EtOH group responded with bradycardia that persisted for several trials whereas the control groups (sham and unhandled) displayed virtually no heart rate responding throughout the test.
DISCUSSION
The purpose of this experiment was to further explore the reported deficits in rate of orienting response habituation resulting from neonatal ethanol exposure (Hunt and Morasch, 2004; Hunt and Phillips, 2004). The results indicate that subjects exposed to ethanol on PD 4-9 and tested 1–2 weeks later (on PD 16 or 23) displayed comparable heart rate orienting responses to the novel olfactory stimulus, but exhibited deficits in habituation of this response, relative to sham-intubated controls. When tested at 30 days of age, however, control subjects (both sham-treated and unhandled) failed to exhibit an orienting response, whereas ethanol-exposed subjects did. Data from 30-day-old animals are difficult to interpret because the control groups did not show the expected orienting response. Nonetheless, these findings suggest that ethanol may have lead to an altered pattern of responding that is not age-typical, or perhaps to a delay in development of the normal response pattern to this novel stimulus (Riley, 1990). However, because subjects were not tested at ages older than 30 days, these issues remain speculative.
Animals showed comparable magnitudes of the heart rate orienting response on the first presentation of the olfactory stimulus, with the exception of the 30-day-old control groups that showed no significant deviation from baseline. Animals in each of the other groups exhibited a decrease in heart rate that peaked at 50–60 beats-per-minute below baseline. The finding that ethanol-exposed subjects did not differ from controls in the relative magnitude or onset latency of the orienting response when tested at 16- or 23-days of age is commensurate with the human data reported by Streissguth et al. (1983). In that report, newborn ethanol-exposed and non-exposed infants were assessed using the Brazelton Neonatal Assessment Scale. While ethanol-induced deficits in rate of habituation of the orienting response to auditory and visual stimuli were evident in exposed infants, these authors reported no differences in initial orientation level. This pattern of results was similarly observed here and in previous reports from our lab (Hunt and Morasch, 2004; Hunt and Phillips, 2004). Others, however (e.g. Coles et al., 1987) have reported changes in orientation and arousal levels in infants prenatally exposed to alcohol.
The finding that the adolescent control subjects did not exhibit orienting bradycardia to the olfactory stimulus is rather perplexing. Previous research has shown that preweanling (12–18 days of age), juvenile (21–23 days of age) and adult (60–90 days of age) rats all exhibit substantial decreases in heart rate to this same stimulus (Hunt et al., 1997a, 1997b; Hunt and Phillips, 2004; Sananes et al., 1988; Siegel et al., 1987). In these studies, however, adolescents were not tested. While, as a group, adolescent-aged controls in both the Sham and Unhandled conditions failed to exhibit an orienting response defined by bradycardia (Graham and Clifton, 1966), individual animals did exhibit heart rate changes to the first presentation of the stimulus. Some of the control animals did indeed respond with orienting bradycardia (n = 6; peak change = −50.7 BPM), but the majority of the animals (n = 15) responded with tachycardia (peak change = +50.7 BPM). Graham (1992) has defined such an increase in heart rate as a defensive response (see also Hunt et al., 1994). Spear (2000) has noted that adolescents sometimes exhibit hyper-responsiveness to novel stimuli compared with either younger or older individuals, while at the same time exhibiting increased sensation-seeking and risk-taking behaviors (e.g. Stansfield and Kirstein, 2006). Perhaps the tachycardia observed in the preponderance of the adolescent control subjects tested here represents this type of enhanced reactivity to novelty. Regardless, it is interesting to note that the alcohol-exposed animals failed to show this age-typical pattern, with the majority of the animals tested (7 out of 8) responding with bradycardia. This might suggest that early alcohol exposure is producing an attenuated stress/defensive response in the adolescent animals (cf. Weinberg et al., 2008) or inducing a developmental delay in responding. It is possible that ethanol-treated subjects tested at a slightly older age would exhibit the tendency toward tachycardia that is evident in the 30-day-old control groups.
The neuroanatomical basis for the observed ethanol-induced deficit in olfactory response habituation is not clear. One difficulty in identifying a brain locus for ethanol’s effects is that the anatomical substrates for olfactory habituation have not been well defined. However, there are some published findings that may shed some light on this issue. First, the main olfactory bulb itself could be an important site for olfactory habituation (for review see Wilson and Linster, 2008). McNamara et al. (2008) for example, reported that habituation to olfactory cues could occur in either the main olfactory bulb or the piriform cortex, depending on the temporal patterns of stimulus presentation. Specifically, habituation on a short time-scale was found to be dependent on changes in neural activity within the piriform cortex. Here, decreases in neural excitability were evident following repeated presentations of a 20 s odorant with relatively short inter-stimulus intervals (ISI; 10 s). In contrast, long-term habituation to olfactory cues was found to involve synaptic depression in mitral cells within the main olfactory bulb. The sequence of stimulus presentations required for this long-term adaptation involved longer odorant exposures (50 s) separated by relatively long (5 min) ISIs. The latter seems more analogous to the stimulus conditions of the present experiment. Notably, changes in olfactory bulb morphology and function have been reported in rats exposed to ethanol during either the prenatal or postnatal periods (Barron and Riley, 1992; Bonthius and West, 1991; Nyquist-Battie and Gochee, 1985; Rockwood and Riley, 1990). Of particular importance are data by Maier et al. (1999) indicating a significant reduction in mitral cell numbers in the main olfactory bulb in rats exposed to ethanol during the neonatal period using a model very similar to the one employed in the present research. We know of no studies that have examined neonatal ethanol-induced alterations in the piriform cortex.
The demonstration that habituation may occur in olfactory-specific pathways (Wilson and Linster, 2008) may clarify previous data on response habituation in this animal model. Hunt and Morasch (2004) for example, not only found that neonatal ethanol resulted in robust deficits in olfactory habituation, they further reported no effect of ethanol exposure on rate of habituation to a simple auditory cue. Kelly and Richards (1998) reported a similar finding, as well as showing that habituation to visual cues was unaffected by neonatal ethanol exposure. These data are somewhat surprising given that humans with a diagnosis of FAS or Fetal Alcohol Spectrum Disorder (FASD) demonstrate deficits in both auditory and visual domains (e.g. Coles et al., 2002; Connor et al., 1999; Streissguth et al., 1983). The failure to observe auditory and visual deficits following neonatal ethanol suggests that the administration of ethanol in this particular fashion is insufficient to compromise the functional integrity of regions involved in the processing or habituation of the orienting response to stimuli within these modalities. Neonatal ethanol administration does appear, however, to render olfactory pathways less able to support sensory and behavioral adaptation to repeated odor presentation. While the modality specificity of our findings places some limits on the generalizability of the data to the human condition, it is noteworthy that many researchers have argued that the olfactory system in rodents may be comparable in complexity to auditory and visual functioning in humans (e.g. Kirstein et al., 1997; Otto & Eichenbaum, 1992a, b). Moreover, olfactory function in individuals with FASD has not, to our knowledge, been examined. Because many developmental and neurological conditions have been associated with decreased olfactory perception and identification, including Alzheimer’s Disease (Devanand et al., 2008), Parkinson’s Disease (Bohnen et al., 2008), schizophrenia (Compton et al., 2006) and attention deficit hyperactivity disorder (Karsz et al., 2008), it is very possible that individuals with FASD would also exhibit olfactory deficits. This remains an intriguing question that awaits further research.
In addition to probable changes in the olfactory bulb, the hippocampal formation may be involved in the ethanol-induced deficits in olfactory habituation. Modality-specific processing within this structure does occur (e.g. Barnet & Hunt, 2005; Rudy & Morledge, 1994; Sakurai, 1996) and indeed the hippocampus may play a particularly important role in olfactory processing (cf. Vanderwolf, 2001). The hippocampus receives olfactory information via the lateral entorhinal cortex and is known to be affected by neonatal ethanol exposure (Barnes and Walker, 1981; Livy et al., 2003; Miller, 1995; Savage and Swartzwelder, 1992). Animals with hippocampal lesions exhibit reduced habituation in an open field and novel object exploration (e.g. Honey et al., 2007). There has been little work to date on the role of the hippocampus and surrounding regions in habituation paradigms that do not use visuospatial cues, however, so generalizations to the olfactory domain must be made with caution. Nonetheless, Staubli et al. (1984) reported that lesions of the lateral entorhinal cortex in adult rats caused rapid forgetting of olfactory information in a two-odor discrimination procedure, and this effect was found to be delay-dependent. Lesioned animals that were trained to discriminate odors separated by intervals of up to 2 min were relatively unimpaired compared with controls. However, performance of the lesioned group dropped markedly when the interval between odor presentations was extended to 3 min or longer. In the present experiment, the ISI separating presentations of the olfactory cue averaged more than 3 min, and the impairment in habituation observed could reflect rapid forgetting of the olfactory cue from trial to trial. If this is correct, and in light of the Staubli et al. (1984) findings, it is possible that within-session habituation in ethanol-exposed animals might be observed with the use of shorter ISIs (Wilson and Linster, 2008; see also Nagahara and Handa, 1997). Experiments designed to address habituation in ethanol-treated animals as a function of ISI duration are currently underway.
The third-trimester animal model of FASD provides an important tool for understanding ethanol’s effects on brain and behavioral development. However, the ethanol-treated subjects in the present experiment also exhibited reduced body weights throughout the neonatal and postweanling periods. It is therefore possible that undernutrition at least partially contributed to the observed effects on olfactory habituation. There are numerous reports of undernutrition affecting brain development, many of which also note severe impairments in several types of learning and memory tasks (Kar et al., 2008; Wenk, 1992). For example, undernutrition can result in altered hippocampal structure (Lister et al., 2005) and function (e.g. Goodlett et al., 1986; Jordan et al., 1981; Rudy & Castro, 1990). There are also reports of increased sensory reactivity (Alamy et al., 2005; Lester et al., 1975). These results are not ubiquitous, however (Campbell & Bedi, 1989; Hall, 1984; Lester, 1975). In the present experiment the ethanol-treated animals weighed less than controls beginning on PD 5, and this reduction in weight persisted throughout testing; therefore undernutrition could be a confounding factor in the memory impairments observed (Weinberg, 1984). Better control over body weights by using a different vehicle solution or through the administration of more supplements during the period of ethanol administration would help to determine the unique contribution of ethanol versus undernutrition on habituation impairments.
Collectively, these results suggest that attention and memory systems required for orienting response habituation are vulnerable to third trimester ethanol insult and, further, that this relatively simple measure can reveal abnormalities that persist at least into the juvenile period (23 days) and possibly also longer, in this rodent model. Habituation is a fundamental aspect of more complex forms of attention, learning and memory as it allows the organism to filter out extraneous stimulation and promotes focus and vigilance on the task at-hand. As discussed previously, rate of habituation and simple recognition memory performance in infancy also predict later cognitive development, including performance on tests of intelligence. Thus habituation paradigms could be particularly useful for further studies of developmental deficits in cognitive functioning arising from early ethanol exposure.
Footnotes
This research was supported by grants from the National Institute on Alcohol Abuse and Alcoholism (AA015343) and the Virginia Tobacco Settlement Foundation.
References
- Alamy M, Errami M, Taghzouti K, Saddiki-Traki F, Bengelloun WA. Effects of postweaning undernutrition on exploratory behavior, memory and sensory reactivity in rats: Implication of the dopaminergic system. Physiol Behav. 2005;86:195–202. doi: 10.1016/j.physbeh.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Barnes DE, Walker DW. Prenatal ethanol exposure permanently reduces the number of pyramidal neurons in rat hippocampus. Dev Brain Res. 1981;1:333–340. doi: 10.1016/0165-3806(81)90071-7. [DOI] [PubMed] [Google Scholar]
- Barnet RC, Hunt PS. Trace and long-delay fear conditioning in the developing rat. Learn Behav. 2005;33:437–443. doi: 10.3758/bf03193182. [DOI] [PubMed] [Google Scholar]
- Barron S, Riley EP. The effects of prenatal alcohol exposure on behavioral and neuroanatomical components of olfaction. Neurotoxicol Teratol. 1992;14:291–297. doi: 10.1016/0892-0362(92)90009-y. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Studenski SA, Constantine GM, Moore RY. Diagnostic performance of clinical motor and non-motor tests of Parkinson disease: A matched case-control study. Eur J Neurol. 2008;15:685–691. doi: 10.1111/j.1468-1331.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Acute and long-term neuronal deficits in the rat olfactory bulb following alcohol exposure during the brain growth spurt. Neurotoxicol Teratol. 1991;13:611–619. doi: 10.1016/0892-0362(91)90044-w. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Information processing (habituation) in infancy and stability in cognitive development. Human Dev. 1989;32:129–136. [Google Scholar]
- Brown RT, Coles CD, Smith IE, Platzman KA, Silverstein J, Erickson S, Falek A. Effects of prenatal alcohol exposure at school age: II. Attention and behavior. Neurotoxicol Teratol. 1991;13:369–376. doi: 10.1016/0892-0362(91)90085-b. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Hayne H, Richardson R. Attention and information processing in infants and adults. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Campbell LF, Bedi KS. The effect of undernutrition during early life on spatial learning. Physiol Behav. 1989;45:883–890. doi: 10.1016/0031-9384(89)90210-2. [DOI] [PubMed] [Google Scholar]
- Carmichael Olson H, Feldman JJ, Streissguth AP, Sampson PD, Bookstein FL. Neuropsychological deficits in adolescents with fetal alcohol syndrome: Clinical findings. Alcohol Clin Exp Res. 1998;22:1998–2012. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Lynch ME, Freides D. Auditory and visual sustained attention in adolescents prenatally exposed to alcohol. Alcohol Clin Exp Res. 2002;26:263–271. [PubMed] [Google Scholar]
- Coles CD, Platzman KA, Raskin-Hood CL. A comparison of children affected by prenatal alcohol exposure and attention deficit, hyperactivity disorder. Alcohol Clin Exp Res. 1997;21:150–161. [PubMed] [Google Scholar]
- Coles CD, Smith IE, Falek A. Prenatal alcohol exposure and infant behavior: Immediate effects and implications for later development. Adv Alcohol Subst Abuse. 1987;6:87–104. doi: 10.1300/J251v06n04_07. [DOI] [PubMed] [Google Scholar]
- Compton MT, Mack LM, Esterberg ML, Bercu Z, Kryda AD, Quintero L, Weiss PS, Walker EF. Associations between olfactory identification and verbal memory in patients with schizophrenia, first-degree relatives, and non-psychiatric controls. Schizophr Res. 2006;86:154–166. doi: 10.1016/j.schres.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Connor PD, Streissguth AP. Effects of prenatal exposure to alcohol across the lifespan. Alcohol Res Health. 1999;20:170–174. [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Streissguth AP, Sampson PD, Bookstein FL, Barr HM. Individual differences in auditory and visual attention among fetal alcohol-affected adults. Alcohol Clin Exp Res. 1999;23:1395–1402. [PubMed] [Google Scholar]
- Devanand DP, Liu X, Tabert MH, Pradhaban G, Cuasay K, Bell K, de Leon MJ, Doty RL, Stern Y, Pelton GH. Combining early markers strongly predicts conversion from mild cognitive impairment to Alzheimer’s disease. Biol Psychiatry. 2008;64:871–879. doi: 10.1016/j.biopsych.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. Adv Infancy Res. 1983;2:31–78. [Google Scholar]
- Goodlett CR, Johnson TB. Neonatal binge ethanol exposure using intubation: Timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Johnson TB. Temporal windows of vulnerability within the third trimester equivalent: Why “knowing when” matters. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol and alcoholism: Effects on brain and development. Erlbaum; Mahwah, NJ: 1999. pp. 59–91. [Google Scholar]
- Goodlett CR, Valentino ML, Morgane PJ, Resnick O. Spatial cue utilization in chronically malnourished rats: Task-specific learning deficits. Dev Psychobiol. 1986;19:1–15. doi: 10.1002/dev.420190102. [DOI] [PubMed] [Google Scholar]
- Graham FK. Attention: The heart beat, the blink and the brain. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Erlbaum; Hillsdale, NJ: 1992. pp. 3–29. [Google Scholar]
- Graham FK, Clifton RK. Heart-rate change as a component of the orienting response. Psychol Bull. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Hall RD. Acoustic startle responses of protein malnourished rats. Physiol Behav. 1984;32:175–181. doi: 10.1016/0031-9384(84)90126-4. [DOI] [PubMed] [Google Scholar]
- Honey RC, Marshall VJ, McGregor A, Futter J, Good M. Revising places past: Sensitization of exploratory activity in rats with hippocampal lesions. Q J Exp Psychol. 2007;60:625–634. doi: 10.1080/17470210601155252. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Hess MF, Campbell BA. Autonomic mediation of unconditioned and conditioned heart rate responses in the 16-day-old rat. Psychobiol. 1994;22:209–218. [Google Scholar]
- Hunt PS, Hess MF, Campbell BA. Conditioned cardiac and behavioral response topography to an olfactory CS dissociates with age. Anim Learn Behav. 1997a;25:53–61. [Google Scholar]
- Hunt PS, Morasch KC. Modality-specific impairments in orienting response habituation following neonatla binge ethanol exposure. Neurotoxicol Teratol. 2004;26:451–459. doi: 10.1016/j.ntt.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Phillips JS. Postnatal binge ethanol exposure affects habituation of the cardiac orienting response to an olfactory stimulus in preweanling rats. Alcohol Clin Exp Res. 2004;28:123–130. doi: 10.1097/01.ALC.0000108650.02216.1A. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Richardson R, Hess MF, Campbell BA. Emergence of conditioned cardiac responses to an olfactory CS paired with an acoustic startle UCS during development: Form and autonomic origins. Dev Psychobiol. 1997b;30:151–163. [PubMed] [Google Scholar]
- Jordan TC, Cane SE, Howells KF. Deficits in spatial memory performance induced by early undernutrition. Dev Psychobiol. 1981;14:317–325. doi: 10.1002/dev.420140404. [DOI] [PubMed] [Google Scholar]
- Kar BR, Rao SL, Chandramouli BA. Cognitive development in children with chronic protein energy malnutrition. Behav Brain Funct. 2008;4:31. doi: 10.1186/1744-9081-4-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsz FR, Vance A, Anderson VA, Brann PG, Wood SJ, Pantelis C, Brewer MJ. Olfactory impairments in child attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2008;69:1462–1468. doi: 10.4088/jcp.v69n0914. [DOI] [PubMed] [Google Scholar]
- Kavšek M. Predicting later IQ from infant visual habituation and dishabituation: A meta-analysis. Appl Dev Psychol. 2004;25:369–393. [Google Scholar]
- Kelly SJ, Richards JE. Heart rate orienting and respiratory sinus arrhythmia development in rats exposed to alcohol or hypoxia. Neurotoxicol Teratol. 1998;20:193–202. doi: 10.1016/s0892-0362(97)00090-1. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and analysis: A researcher’s handbook. 2. Prentice-Hall; Englewood Cliffs, NJ: 1982. [Google Scholar]
- Kerns KA, Don A, Mateer CA, Streissguth AP. Cognitive deficits in nonretarded adults with fetal alcohol syndrome. J Learn Disabil. 1997;30:685–693. doi: 10.1177/002221949703000612. [DOI] [PubMed] [Google Scholar]
- Kirstein CL, Philpot R, Dark T. Fetal alcohol syndrome: Early olfactory learning as a model system to study neurobehavioral deficits. Int J Neurosci. 1997;89:119–132. doi: 10.3109/00207459708988467. [DOI] [PubMed] [Google Scholar]
- Kodituwakku PW. Defining the behavioral phenotype in children with fetal alcohol spectrum disorders: A review. Neurosci Biobehav Rev. 2007;31:192–201. doi: 10.1016/j.neubiorev.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Korkman M, Kettunen S, Autti-Ramo I. Neurocognitive impairment in early adolescence following prenatal alcohol exposure of varying duration. Child Neuropsychol. 2003;9:117–128. doi: 10.1076/chin.9.2.117.14503. [DOI] [PubMed] [Google Scholar]
- Lacey JI, Lacey BC. Some autonomic-central nervous system interrelationships. In: Black P, editor. Physiological correlates of emotion. Academic Press; NY: 1970. pp. 205–227. [Google Scholar]
- Lang PJ, Simons RF, Balaban M. Attention and orienting: Sensory and motivational processes. Erlbaum; Mahwah, NJ: 1997. [Google Scholar]
- Lester BM. Cardiac habituation of the orienting response to an auditory signal in infants of varying nutritional status. Dev Psychol. 1975;11:432–442. [Google Scholar]
- Lester BM, Klein RE, Martinez SJ. The use of habituation in the study of the effects of infantile malnutrition. Dev Psychobiol. 1975;8:541–546. doi: 10.1002/dev.420080611. [DOI] [PubMed] [Google Scholar]
- Lister JP, Blatt GJ, DeBassio WA, Kemper TL, Tonkiss J, Galler JR, Rosene DL. Effect of prenatal protein malnutrition on numbers of neurons in the principal cell layers of the adult rat hippocampal formation. Hippocampus. 2005;15:393–403. doi: 10.1002/hipo.20065. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Miller EK, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: Effects of binge-like alcohol exposure on the developing rat hippocampus. Neurotoxicol Teratol. 2003;25:447–458. doi: 10.1016/s0892-0362(03)00030-8. [DOI] [PubMed] [Google Scholar]
- Maier SE, Miller JA, Blackwell JM, West JR. Fetal alcohol exposure and temporal vulnerability: Regional differences in cell loss as a function of the timing of binge-like alcohol exposure during brain development. Alcohol Clin Exp Res. 1999;23:726–734. doi: 10.1111/j.1530-0277.1999.tb04176.x. [DOI] [PubMed] [Google Scholar]
- McCall RB. What process mediates predictions of childhood IQ from infant habituation and recognition memory? Speculations on the roles of inhibition and rate of information processing. Intelligence. 1994;18:107–125. [Google Scholar]
- McCall RB, Carriger MS. A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Dev. 1993;64:57–79. [PubMed] [Google Scholar]
- McNamara AM, Magidson PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn Mem. 2008;15:117–125. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res. 1995;19:1500–1509. doi: 10.1111/j.1530-0277.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Nagahara AH, Handa RJ. Fetal alcohol exposure produces delay-dependent memory deficits in juvenile and adult rats. Alcohol Clin Exp Res. 1997;21:710–715. [PubMed] [Google Scholar]
- Nanson JL, Hiscock M. Attention deficits in children exposed to alcohol prenatally. Alcohol Clin Exp Res. 1990;14:656–661. doi: 10.1111/j.1530-0277.1990.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Nyquist-Battie C, Gochee A. Alterations in the development of the main olfactory bulb of the mouse after ethanol exposure. Int J Dev Neurosci. 1985;3:211–222. doi: 10.1016/0736-5748(85)90026-7. [DOI] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H. Olfactory learning and memory in the rat: A “model system” for studies of the neurobiology of memory. In: Serby MJ, Chobor KL, editors. Science of olfaction. Springer-Verlag; NY: 1992a. pp. 213–244. [Google Scholar]
- Otto T, Eichenbaum H. Toward a comprehensive account of hippocampal function: Studies of olfactory learning permit an integration of data across multiple levels of neurobiological analysis. In: Squire LR, Butters N, editors. Neuropsychology of memory. Guilford; NY: 1992b. pp. 415–428. [Google Scholar]
- Richardson R, Campbell BA. Ontogeny of long-term, nonassociative memory in the rat. Animal Learn Behav. 1991;19:1–10. [Google Scholar]
- Riley EP. The long-term behavioral effects of prenatal alcohol exposure in rats. Alcohol Clin Exp Res. 1990;14:670–673. doi: 10.1111/j.1530-0277.1990.tb01225.x. [DOI] [PubMed] [Google Scholar]
- Rockwood GA, Riley EP. Nipple attachment behavior in rat pups exposed to alcohol in utero. Neurotoxicol Teratol. 1990;12:383–389. doi: 10.1016/0892-0362(90)90058-k. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF. Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Dev Psychol. 1995;31:685–696. [Google Scholar]
- Rudy JW, Castro CA. Undernutrition during the brain growth period of the rat significantly delays the development of processes mediating Pavlovian trace conditioning. Behav Neural Biol. 1990;53:307–320. doi: 10.1016/0163-1047(90)90170-b. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Morledge P. Ontogeny of contextual fear conditioning in rats: Implications for consolidation, infantile amnesia and hippocampal system function. Behav Neurosci. 1994;108:227–234. doi: 10.1037//0735-7044.108.2.227. [DOI] [PubMed] [Google Scholar]
- Saiers JA, Richardson R, Campbell BA. Disruption and recovery of the orienting response following shock or context change in preweanling rats. Psychophysiology. 1990;27:45–56. doi: 10.1111/j.1469-8986.1990.tb02177.x. [DOI] [PubMed] [Google Scholar]
- Sakurai Y. Hippocampal and neocortical cell assemblies encode memory processes for different types of stimuli in the rat. J Neurosci. 1996;16:2809–2819. doi: 10.1523/JNEUROSCI.16-08-02809.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananes CB, Gaddy JR, Campbell BA. Ontogeny of conditioned heart rate to an olfactory stimulus. Dev Psychobiol. 1988;21:117–133. doi: 10.1002/dev.420210202. [DOI] [PubMed] [Google Scholar]
- Savage DD, Swartzwelder HS. Effects of perinatal ethanol exposure on hippocampal formation. In: Watson RR, editor. Alcohol and neurobiology: Brain development and hormone regulation. CRC Press; Boca Raton, FL: 1992. pp. 171–200. [Google Scholar]
- Siegel MA, Sananes CB, Gaddy JR, Campbell BA. Dissociation of heart rate and somatomotor orienting responses to novel stimuli in preweanling rats. Psychobiol. 1987;15:122–127. [Google Scholar]
- Sokolov EN. Perception and the conditioned reflex. Macmillan; NY: 1963. [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stansfield KH, Kirstein CL. Effects of novelty on behavior in the adolescent and adult rat. Dev Psychobiol. 2006;48:10–15. doi: 10.1002/dev.20127. [DOI] [PubMed] [Google Scholar]
- Staubli U, Ivy G, Lynch G. Hippocampal denervation causes rapid forgetting of olfactory information in rats. Proc Natl Acad Sci. 1984;81:5885–5887. doi: 10.1073/pnas.81.18.5885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streissguth AP. Offspring effects of prenatal alcohol exposure from birth to 25 years: The Seattle Prospective Longitudinal Study. J Clin Psychol Med Settings. 2007;14:81–101. [Google Scholar]
- Streissguth AP, Barr HM, Martin DC. Maternal alcohol use and neonatal habituation assessed with the Brazelton Scale. Child Dev. 1983;54:1109–1118. [PubMed] [Google Scholar]
- Streissguth AP, Martin DC, Barr HM, Sandman BM, Kirscher GL, Darby BL. Intrauterine alcohol and nicotine exposure: Attention and reaction time in 4-year-old children. Dev Psychol. 1984;20:533–541. [Google Scholar]
- Streissguth AP, Sampson PD, Barr H. Neurobehavioral dose-response effects of prenatal alcohol exposure in humans from infancy to adulthood. Ann N Y Acad Sci. 1989;562:145–158. doi: 10.1111/j.1749-6632.1989.tb21013.x. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. The hippocampus as an olfacto-motor mechanism: Were the classical anatomists right after all? Behav Brain Res. 2001;127:25–47. doi: 10.1016/s0166-4328(01)00354-0. [DOI] [PubMed] [Google Scholar]
- Weinberg J. Nutritional issues in perinatal alcohol exposure. Neurobehav Toxicol Teratol. 1984;6:261–269. [PubMed] [Google Scholar]
- Weinberg J, Sliwowska JH, Lan N, Hellemans KGC. Prenatal alcohol exposure: Foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinology. 2008;20:470–488. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk GL. Dietary factors that influence the neural substrates of memory. In: Isaacson RL, Jensen KF, editors. The vulnerable brain and environmental risks, Vol 1: Malnutrition and hazard assessment. Plenum; NY: 1992. pp. 67–75. [Google Scholar]
- Wilson DA, Linster C. Neurobiology of a simple memory. J Neurophysiol. 2008;100:2–7. doi: 10.1152/jn.90479.2008. [DOI] [PubMed] [Google Scholar]