Abstract
Background
Gingival fibromas and dental pitting are among the diagnostic criteria for tuberous sclerosis complex (TSC).
Objective
Our goal was to document the oral findings in 58 adult patients with TSC.
Results
Forty patients (69%) had oral fibromas, appearing mostly on the attached or interdental gingiva. Other oral sites included buccal and labial mucosa, the superior labial frenulum, palate, and tongue. Fifty-six patients (97 %) had multiple dental enamel pits.
Limitations
This case series comprised predominantly adult females with TSC and lymphangioleiomyomatosis.
Conclusions
Oral fibromas in TSC are mostly, but not exclusively, gingival. Dental pits are present in nearly all patients. The multiple oral papules in TSC may appear similar to those observed in Cowden syndrome, Birt-Hogg-Dube syndrome, and rarely in multiple endocrine neoplasia type 1.
INTRODUCTION
TSC is an autosomal dominant syndrome in which patients develop hamartomas of the brain, heart, kidneys, lungs, and skin. Gingival fibromas and dental pitting constitute two minor features in the diagnostic criteria for TSC1. Most studies emphasize the gingival location for fibromas in TSC, but they also occur at other intra-oral locations2–12. We studied the oral findings in 58 TSC patients to better define the location and appearance of oral fibromas in TSC.
PATIENTS AND METHODS
Adult patients meeting diagnostic criteria for TSC were seen in the Clinical Center at the National Institutes of Health, a tertiary referral center. Patients were recruited for studies of TSC and lymphangioleiomyomatosis (LAM), a pulmonary disease that occurs in adult women with TSC. Patients were either known to have LAM, or were being screened for the presence of LAM. Informed consent was obtained according to protocols approved by the National Heart, Lung, and Blood Institute Institutional Review Board (protocols 00-H-0051, 95-H-0186 and/or 82-H-0032). No children were included since LAM rarely occurs in children. In addition, patients were excluded if they were unable to provide informed consent. All patients were from different families, except for one pair of affected siblings. Fifty-eight patients had skin and oral examinations and photography. All patients were evaluated by at least one author (TND). The dermatological consult notes and all oral photographs were retrospectively tabulated by three authors (JDS, C-HH, and TND). Mucosal biopsies were obtained on five lesions from four patients.
RESULTS
The patients ranged in age from 23 to 69 years, with a mean age of 40 years (standard deviation 10 years). Forty-six of the 58 patients (79%) had lymphangioleiomyomatosis (LAM), a TSC-associated pulmonary disease observed primarily in women13. Because of the bias towards TSC patients with LAM, this study included fifty-five females and three males.
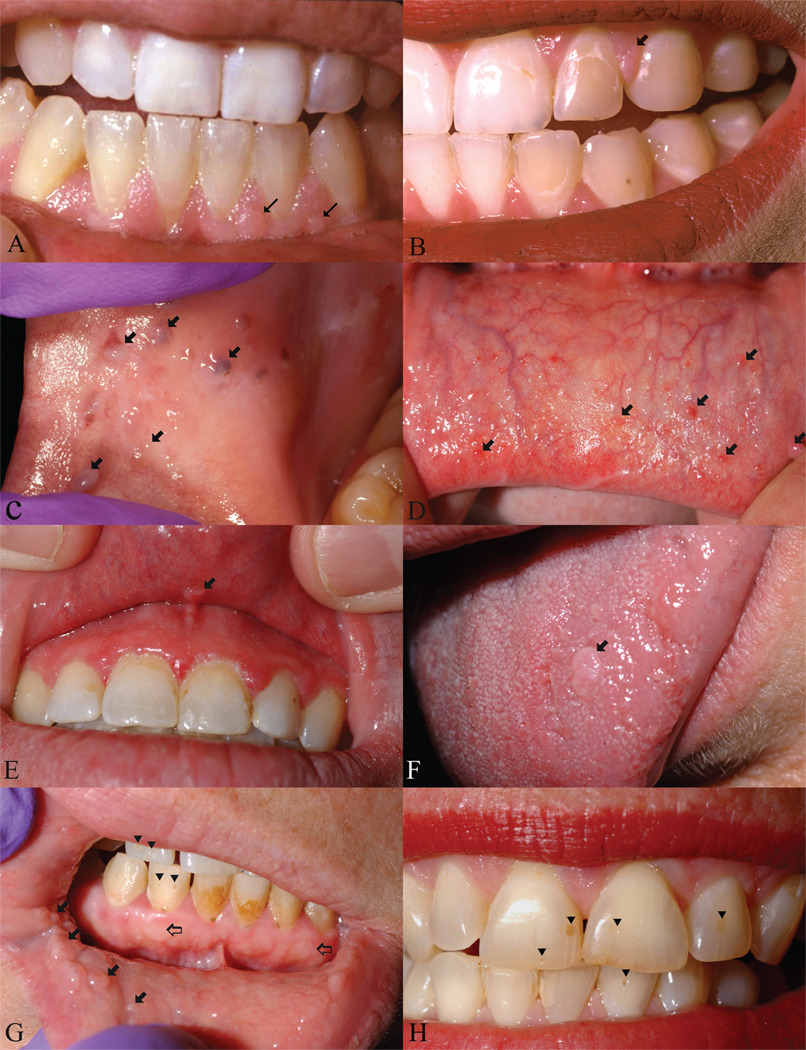
The locations and frequencies of oral fibromas are shown in Table I. Fibromas on the attached gingiva were dome-shaped papules of the same color as the gingiva or slightly whitish (Figure 1A). Some appeared as multiple discrete, small (1–2 mm) pink or whitish pink clustered papules with a papillomatous appearance. Interdental fibromas were protrusions of the interdental papillae, occasionally with irregular small papules on the surface (Figure 1B). Nongingival oral fibromas on the buccal mucosa (Figure 1C) and labial mucosa (Figure 1D) were the color of oral mucosa or slightly yellowish, and had a bluish hue in some darkly pigmented individuals. The surface was glistening, often with telangiectases appearing as a red blush or extending in a brush-like pattern from one side of the base to the tip. Five patients displayed a discrete mucosal papule on the mid-line superior labial frenulum which, to our knowledge, has not been previously reported in TSC (Figure 1E). Fibromas on the anterior dorsal or lateral tongue were few in number and were dome-shaped or oval papules measuring about 5 mm diameter (Figure 1F). Multiple dental enamel pits ranged from pinpoint, ice-pick lesions to larger indentations up to 3 mm diameter (Figure 1G&H).
Table I.
| Gingival Fibromas | 30/58 | 52 % |
| Attached Gingival | 23/58 | 40 % |
| Interdental Gingival | 10/58 | 17 % |
| Fibromas at other oral sites | 23/58 | 40 % |
| Buccal | 14/58 | 24 % |
| Labial | 10/58 | 17 % |
| Superior Labial Frenulum | 5/58 | 9 % |
| Hard Palate | 2/58 | 3 % |
| Tongue | 2/58 | 3 % |
| Oral Fibromas at any site | 40/58 | 69 % |
| Gingival Overgrowth | 4/58 | 7 % |
| Dental Pits | 56/58 | 97 % |
Figure 1.
Oral findings in TSC. A. Attached gingival fibromas; B. Interdental gingival fibromas; C. Buccal fibromas; D. Labial mucosal fibromas; E. Superior labial frenulum fibroma; F. Tongue fibroma; G. Labial and angular commissure fibromas, gingival fibromas (open arrows) and dental pits (arrowheads); H. Multiple dental enamel pits (arrowheads). Fibromas marked with closed arrows except as indicated.
Numbers of oral fibromas per patient ranged from 1 to 15, median 5, totaling two or more in 32 patients. Oral fibromas were asymptomatic. Many patients were unaware of their presence, so age of onset is unknown.
Diffuse gingival overgrowth was observed in four patients. Three of these had histories of infantile spasm or seizure, but none had been treated with anti-convulsive drugs. The extent of gingival overgrowth was mild, and oral hygiene was good. In one patient, gingival overgrowth was associated with mouth breathing. Another patient was taking nifedipine, a drug associated with gingival overgrowth14.
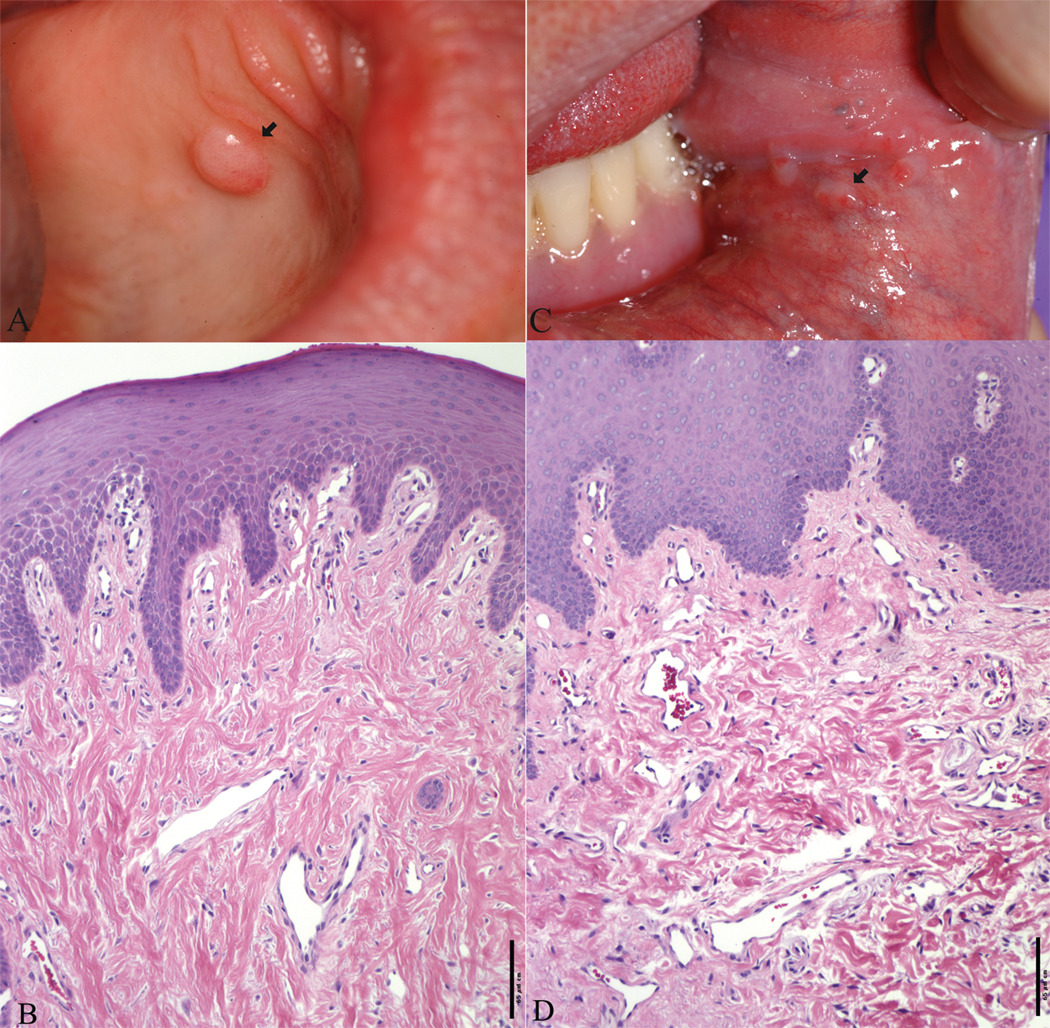
Four biopsy specimens of oral fibromas were obtained from three patients, including the left inner lower lip, the hard palate (Figure 2A) and left buccal mucosa (Figure 2C), and the right buccal mucosa. These showed findings of fibromas, with benign squamous epithelium and a vascularized fibrous stroma (Figures 2B and D). In addition, one patient had a biopsy of a solitary pharyngeal lesion. This showed oral mucosa with underlying lymphoid tissue and florid follicular hyperplasia. It is not clear if this is related to TSC.
Figure 2.
Clinical and histological features of oral lesions in one TSC patient. Biopsy of a dome-shaped fibroma on the hard palate (A) revealed a haphazard array of collagen with several thin-walled vascular channels underlying a thickened epithelium (B). Biopsy of a fibroma at the angular commissure (C) revealed vessels and fibrous bands of collagen (D). (B and D stained with H&E, bar = 65µm).
DISCUSSION
Oral fibromas are common in adults with TSC. We found them in 69% of 58 patients, a frequency higher than those in previous studies, in which oral fibromas were observed in 56% of 39 TSC patients4 and in 36% of 131 TSC patients12. The higher percentage in our study may due to differences in ages of the study populations, since oral fibromas appear to be less common in children than adults. Only five of 37 children (8%) under age 11 had oral fibromas12.
Fibromas were gingival in 30 patients (52%) and at other oral sites in 23 patients (40%). The locations of oral fibromas in non-gingival sites, in descending order of frequency, were buccal mucosa (most commonly just inside the angular commissure), labial mucosa, superior labial frenulum, and palate and tongue. TSC oral fibromas were multiple in numbers and often occurred at more than one site, as previously reported2–12.
Oral fibromas also can occur sporadically in the general population, but at a much lower frequency. The prevalence of oral fibromas was 12/1,000 in a survey of 23,616 individuals over 35 years of age 15. Sporadic oral fibromas may be similar in appearance to TSC oral fibromas, but are typically single or few. They are associated with repeated trauma, and are often termed irritation fibromas. The most common locations are on the tongue and buccal mucosa16,17.
We suggest that the TSC diagnostic criterion pertaining to oral fibromas should be expanded to include multiple oral fibromas at gingival and non-gingival sites. In this study, 30 patients had at least one gingival fibroma and 40 had at least one oral fibroma at any site. Requiring the presence of multiple lesions, as is the case for angiofibromas, would reduce the number of patients fulfilling this criterion to 32. One patient had multiple oral fibromas and no angiofibromas or periungual fibromas, suggesting that oral fibromas may be significant for diagnosis in some cases.
Several familial tumor syndromes can present with multiple facial and oral papules, including TSC, Cowden syndrome18, Birt-Hogg-Dube syndrome19, and multiple endocrine neoplasia type I20. These conditions can be distinguished from TSC by absence of dental pitting, characteristic skin lesions, and associated internal findings. Oral manifestations of Cowden syndrome are typically more extensive than TSC, but can be clinically and histologically similar21. We hypothesize that the similarities between oral mucosal lesions in Cowden syndrome and TSC are related to dysregulation of the mTOR signaling pathway. Loss of PTEN function in Cowden syndrome and loss of TSC1/TSC2 function in TSC both result in increased activation of mTOR22,23. It is not known whether loss of function of the BHD gene in oral lesions dysegulates signaling downstream of mTOR.
Gingival overgrowth, the term currently preferred over gingival hyperplasia or gingival hypertrophy14, can be drug induced in TSC8, especially by phenytoin which causes gingival overgrowth in 10–50% of non-TSC patients14. However, gingival overgrowth in TSC may occur in the absence of phenytoin treatment4,24, and four patients in the current study had never been treated with anticonvulsants. Gingival overgrowth may be treated by withdrawal or replacement of medication when that is possible. Gingivectomy has been used in severe cases, but recurrent disease has been observed24,25.
Dental enamel pitting is observed in up to 100% of TSC patients26–29. Dental pits can also be observed in the general population, but at lower frequency and with fewer lesions than in TSC26. Enamel defects are also observed in pitted hypoplastic amelogenesis imperfecta, Vitamin D dependent rickets, pseudohypoparathyroidism, and junctional epidermolysis bullosa. The occurrence of pits in normal individuals and other diseases limits the use of this sign in the diagnostic criteria to a minor feature1.
In conclusion, this study demonstrates that oral fibromas in TSC are mostly but not exclusively gingival. We propose that multiple oral fibromas, rather than gingival fibromas, be used as a minor feature for diagnosis. Dental pitting, another minor feature among the diagnostic criteria, is observed in nearly all individuals with TSC. Other syndromes to consider in the differential diagnosis of patients with multiple facial and oral papules include Cowden syndrome, Birt-Hogg-Dube syndrome, and multiple endocrine neoplasia type I.
ACKNOWLEDGEMENTS
This work was supported by a Clinical Scientist Development Award from the Doris Duke Charitable Foundation, NIH grant R01 CA100907, and the Intramural Research Program of the National Institutes of Health, National Heart, Lung, and Blood Institute. We thank the Tuberous Sclerosis Alliance and The LAM Foundation for patient referral. We thank Drs. Leonard Sperling and Martha Vaughan for helpful discussions and critical review of the manuscript.
Footnotes
Conflict of Interest Disclosure: None Declared.
Disclaimer: The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army, or the Department of Defense.
REFERENCES
- 1.Roach ES, Gomez MR, Northrup H. Tuberous sclerosis complex consensus conference: revised clinical diagnostic criteria. J Child Neurol. 1998;13:624–628. doi: 10.1177/088307389801301206. [DOI] [PubMed] [Google Scholar]
- 2.Lopez E, Escovich L, Vigna A. Tuberous sclerosis: presentation of a clinical case with oral manifestations. Med Oral. 2003;8:122–128. [PubMed] [Google Scholar]
- 3.Lopez-Lopez J, Rodriguez-de-Rivera-Campillo E, Marques-Soares MS, Finestres-Zubeldia F, Chimenos-Kustner E, Rosello-Llabres X. Tuberous sclerosis and its oral manifestations. A clinical case. Med Oral. 2004;9:216–223. [PubMed] [Google Scholar]
- 4.Lygidakis NA, Lindenbaum RH. Oral fibromatosis in tuberous sclerosis. Oral Surg Oral Med Oral Pathol. 1989;68:725–728. doi: 10.1016/0030-4220(89)90162-x. [DOI] [PubMed] [Google Scholar]
- 5.Mackler SB, Shoulars HW, Burkes EJ., Jr Tuberous sclerosis with gingival lesions. Oral Surg Oral Med Oral Pathol. 1972;34:619–624. doi: 10.1016/0030-4220(72)90345-3. [DOI] [PubMed] [Google Scholar]
- 6.Murti PR, Bhonsle RB, Mehta FS, Daftary DK. Oro-facial lesions of tuberous sclerosis. Int J Oral Surg. 1980;9:292–297. doi: 10.1016/s0300-9785(80)80037-8. [DOI] [PubMed] [Google Scholar]
- 7.Papanayotou P, Vezirtzi E. Tuberous sclerosis with gingival lesions. Report of a case. Oral Surg Oral Med Oral Pathol. 1975;39:578–582. doi: 10.1016/0030-4220(75)90199-1. [DOI] [PubMed] [Google Scholar]
- 8.Scully C. Orofacial manifestations in tuberous sclerosis. Oral Surg Oral Med Oral Pathol. 1977;44:706–716. doi: 10.1016/0030-4220(77)90380-2. [DOI] [PubMed] [Google Scholar]
- 9.Scully C. Oral mucosal lesions in association with epilepsy and cutaneous lesions: the Pringle-Bourneville syndrome. Int J Oral Surg. 1981;10:68–72. doi: 10.1016/s0300-9785(81)80010-5. [DOI] [PubMed] [Google Scholar]
- 10.Smith DC, Porter SR, Scully C. Gingival and other oral manifestations in tuberous sclerosis: a case report. Periodontal Clin Investig. 1993;15:13–16. [PubMed] [Google Scholar]
- 11.Tillman HH, De Caro F. Tuberous sclerosis. Oral Surg Oral Med Oral Pathol. 1991;71:301–302. doi: 10.1016/0030-4220(91)90304-u. [DOI] [PubMed] [Google Scholar]
- 12.Webb DW, Clarke A, Fryer A, Osborne JP. The cutaneous features of tuberous sclerosis: a population study. Br J Dermatol. 1996;135:1–5. [PubMed] [Google Scholar]
- 13.Steagall WK, Taveira-DaSilva AM, Moss J. Clinical and molecular insights into lymphangioleiomyomatosis. Sarcoidosis Vasc Diffuse Lung Dis. 2005;22 Suppl 1:S49–S66. [PubMed] [Google Scholar]
- 14.Kataoka M, Kido J, Shinohara Y, Nagata T. Drug-induced gingival overgrowth--a review. Biol Pharm Bull. 2005;28:1817–1821. doi: 10.1248/bpb.28.1817. [DOI] [PubMed] [Google Scholar]
- 15.Bouquot JE, Gundlach KK. Oral exophytic lesions in 23,616 white Americans over 35 years of age. Oral Surg Oral Med Oral Pathol. 1986;62:284–291. doi: 10.1016/0030-4220(86)90010-1. [DOI] [PubMed] [Google Scholar]
- 16.Bataineh A, Al-Dwairi ZN. A survey of localized lesions of oral tissues: a clinicopathological study. J Contemp Dent Pract. 2005;6:30–39. [PubMed] [Google Scholar]
- 17.Ono Y, Takahashi H, Inagi K, Nakayama M, Okamoto M. Clinical study of benign lesions in the oral cavity. Acta Otolaryngol Suppl. 2002:79–84. doi: 10.1080/000164802760057644. [DOI] [PubMed] [Google Scholar]
- 18.Starink TM. Cowden's disease: analysis of fourteen new cases. J Am Acad Dermatol. 1984;11:1127–1141. doi: 10.1016/s0190-9622(84)70270-2. [DOI] [PubMed] [Google Scholar]
- 19.Toro JR, Glenn G, Duray P, Darling T, Weirich G, Zbar B, et al. Birt-Hogg-Dube syndrome: a novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195–1202. doi: 10.1001/archderm.135.10.1195. [DOI] [PubMed] [Google Scholar]
- 20.Darling TN, Skarulis MC, Steinberg SM, Marx SJ, Spiegel AM, Turner M. Multiple facial angiofibromas and collagenomas in patients with multiple endocrine neoplasia type 1. Arch Dermatol. 1997;133:853–857. [PubMed] [Google Scholar]
- 21.Salem OS, Steck WD. Cowden's disease (multiple hamartoma and neoplasia syndrome). A case report and review of the English literature. J Am Acad Dermatol. 1983;8:686–696. doi: 10.1016/s0190-9622(83)70081-2. [DOI] [PubMed] [Google Scholar]
- 22.Abel TW, Baker SJ, Fraser MM, Tihan T, Nelson JS, Yachnis AT, et al. Lhermitte-Duclos disease: a report of 31 cases with immunohistochemical analysis of the PTEN/AKT/mTOR pathway. J Neuropathol Exp Neurol. 2005;64:341–349. doi: 10.1093/jnen/64.4.341. [DOI] [PubMed] [Google Scholar]
- 23.Chan JA, Zhang H, Roberts PS, Jozwiak S, Wieslawa G, Lewin-Kowalik J, et al. Pathogenesis of tuberous sclerosis subependymal giant cell astrocytomas: biallelic inactivation of TSC1 or TSC2 leads to mTOR activation. J Neuropathol Exp Neurol. 2004;63:1236–1242. doi: 10.1093/jnen/63.12.1236. [DOI] [PubMed] [Google Scholar]
- 24.Stirrups DR, Inglis J. Tuberous sclerosis with nonhydantoin gingival hyperplasia. Report of a case. Oral Surg Oral Med Oral Pathol. 1980;49:211–213. doi: 10.1016/0030-4220(80)90048-1. [DOI] [PubMed] [Google Scholar]
- 25.Thomas D, Rapley J, Strathman R, Parker R. Tuberous sclerosis with gingival overgrowth. J Periodontol. 1992;63:713–717. doi: 10.1902/jop.1992.63.8.713. [DOI] [PubMed] [Google Scholar]
- 26.Flanagan N, O'Connor WJ, McCartan B, Miller S, McMenamin J, Watson R. Developmental enamel defects in tuberous sclerosis: a clinical genetic marker? J Med Genet. 1997;34:637–639. doi: 10.1136/jmg.34.8.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho CS, Lin SP, Shen EY, Chiu NC. Enamel pitting in childhood tuberous sclerosis. J Formos Med Assoc. 1995;94:414–417. [PubMed] [Google Scholar]
- 28.Mlynarczyk G. Enamel pitting: a common symptom of tuberous sclerosis. Oral Surg Oral Med Oral Pathol. 1991;71:63–67. doi: 10.1016/0030-4220(91)90523-f. [DOI] [PubMed] [Google Scholar]
- 29.Sampson JR, Attwood D, al Mughery AS, Reid JS. Pitted enamel hypoplasia in tuberous sclerosis. Clin Genet. 1992;42:50–52. doi: 10.1111/j.1399-0004.1992.tb03137.x. [DOI] [PubMed] [Google Scholar]