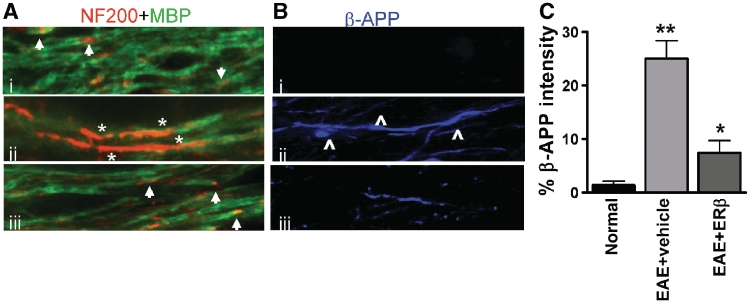
Figure 6.
Decrease in demyelination and axon damage in oestrogen receptor β ligand-treated EAE callosal axons. (A) High magnification confocal images (×60) were taken to identify the presence of demyelination and axon damage. Normal myelinated axons (i) had even myelin basic protein (MBP) immunostaining with small areas that were MBP− and NF200+ and are most likely the nodes of Ranvier (arrow). Vehicle-treated EAE axons (ii) expressed large areas that were MBP− and NF200+ indicative of demyelination (*). Oestrogen receptor β ligand treatment during EAE (iii) had myelinated axons similar to normal. (B) Axon degeneration was assessed with β-amyloid precursor protein accumulation. Unlike the normal control corpus callosum that did not show axonal pathology with β-amyloid precursor protein− immunostaining (blue), vehicle-treated EAE mice had demyelinated axons that showed swelling, beading (⁁) and increased areas of β-amyloid precursor protein accumulation. Oestrogen receptor β treatment during EAE significantly reduced the extent of axon pathology. (C) Quantification of β-amyloid precursor protein immunostaining intensity in the corpus callosum showed nearly 70% less accumulation in oestrogen receptor β ligand-treated EAE compared to vehicle-treated EAE. *P < 0.05; **P < 0.001, ANOVAs; Bonferroni’s multiple comparison post test; n = 5 mice in each treatment group.