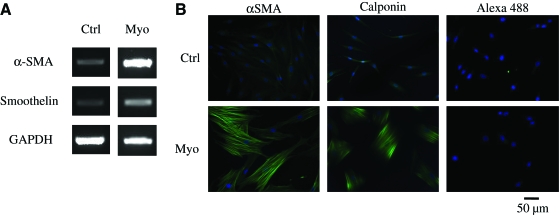
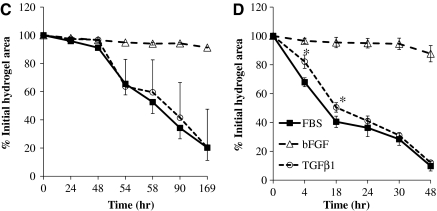
FIG. 5.
HFCs demonstrated myogenic differentiation potential and force generation ability. HFCs were cultured in the myogenic differentiation medium for 1 week. (A) RT-PCR for αSMA, and smoothelin. (B) Immunostaining for αSMA and calponin. Cells stained with Alexa 488 secondary antibody only served as negative control. All samples were counterstained with DAPI to observe the nuclei. Representative images are shown from one out of three independent experiments. (C) HFCs were cultured in the presence of bFGF (2 ng/mL) for 5 days until they reached 85%–90% confluence. At that time the cells were trypsinized and embedded in fibrin that was allowed to polymerize in 24-well plates to form disks. One hour after polymerization, the gels were detached from the walls and allowed to compact in the presence of FBS alone or supplemented with bFGF (2 ng/mL) or TGF-β1 (2 ng/mL). At the indicated times, gels were photographed and their area was measured using ImageJ software. The ratio of gel area at the indicated times over the initial area was plotted as percentage of initial hydrogel area over time. (D) HFCs were cultured in DMEM with 10% FBS alone or supplemented with bFGF (2 ng/mL) or TGF-β1 (2 ng/mL) for 5 days. At that time, the cells were embedded in fibrin hydrogels that were incubated with the medium of the same composition. The percentage of initial hydrogel area was plotted over time. All values represent the mean ± SD of triplicate samples in a representative experiment (n = 3). Asterisks denote p < 0.05 between the TGF-β1- and FBS-treated hydrogels at the same time point. bFGF-treated samples were significantly different (p < 0.05) than either the TGF-β1- or FBS-treated samples at all times. P-αSMA, smooth muscle alpha-actin promoter; TGF-β1, transforming growth factor-beta 1. Color images available online at www.liebertonline.com/ten.