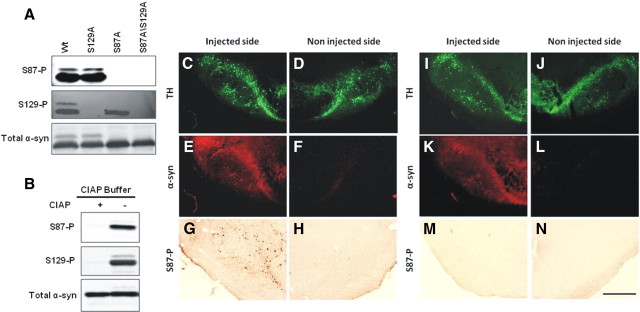
Figure 1.
Specificity of anti-S87-P antibody. A, WT and mutant α-syn phosphorylated for 24 h were separated on a 12%SDS gel and probed with anti-α-syn (211, 1:500), anti-S129-P (1:5000), or anti-S87-P (1:100) α-syn antibodies. B, S87-P and S129-P antibodies failed to detect phosphorylated forms of α-syn treated with calf intestinal alkaline phosphatase. C–N, Virus mediated-overexpression of human α-syn A30P S129A (C–H) and human α-syn A30P S87D S129A (I–N) in the rat substantia nigra. The staining for human α-syn (LB509) colocalized with tyrosine hydroxylase (TH) staining (C, D, I, J) in the injected side (E, K), but was absent in the noninjected side (F, L). The staining for S87-P was restricted to the side injected with the virus coding for human α-syn A30P S129A (G), whereas no staining could be detected on the side injected with the mutant α-syn form S87D (M). Noninjected sides also appeared negative (H, N). Scale bar, 500 μm.