Figure 7.
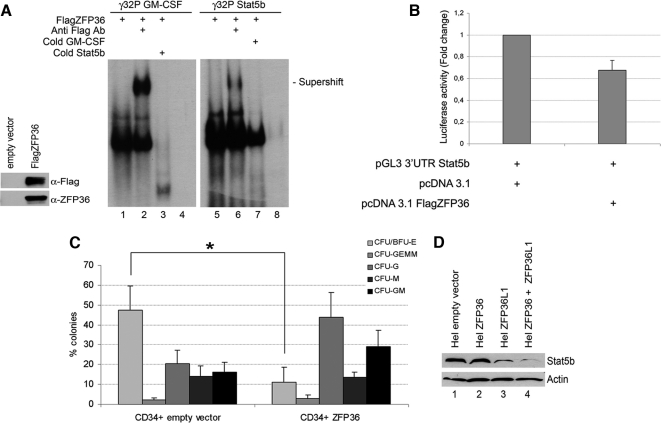
ZFP36 behaves similarly to ZFP36L1 in binding to and destabilizing mRNAs spanning Stat5b 3′UTR and in inhibiting erythroid differentiation of CD34+ HSCs. (A) Left, Western blot demonstrating identity and integrity of in vitro–translated FlagZFP36 protein: immunoblotting was performed with anti-Flag or anti-ZFP36 antibody as indicated. Right, RNA mobility shift assay performed by incubating in vitro–translated FlagZFP36 protein with labeled RNA probes spanning the ARE in the 3′UTR of GM-CSF mRNA (used as a positive control, lanes 1–4) or the ARE in the 3′UTR of Stat5b mRNA (lanes 5–8). Supershifts were obtained by an additional incubation with anti-Flag Ab (lanes 2 and 6). Competition experiments were performed with cold Stat5b probe (lane 3) or with cold GM-CSF probe (lane 7). Lanes 4 and 8 represent labeled probes incubated with an in vitro translation reaction mix performed on empty vector. (B) Luciferase activity assay performed in HEK293 cells transfected with pcDNA3.1 empty expression vector (left) or with pcDNA3.1 overexpressing FlagZFP36 (10 ng; right) together with pGL3 reporter construct encoding for a luciferase gene fused to the 3′UTR of Stat5b. Luciferase activity is represented in terms of fold change; error bars, SEM calculated on a set of five independent experiments. (C) Clonogenic assay performed on CD34+ HSCs transduced with empty vector (left) or overexpressing ZFP36 (right); error bars, SEM calculated on four independent experiments (*p < 0.05). (D) Western blot analysis showing Stat5b levels in Hel cells transfected with empty vector (lane 1), with ZFP36-overexpressing vector (lane 2), with ZFP36L1-overexpressing vector (lane 3), or transfected with both overexpressing vectors (lane 4). Cells were lysed 72 h after transfection.