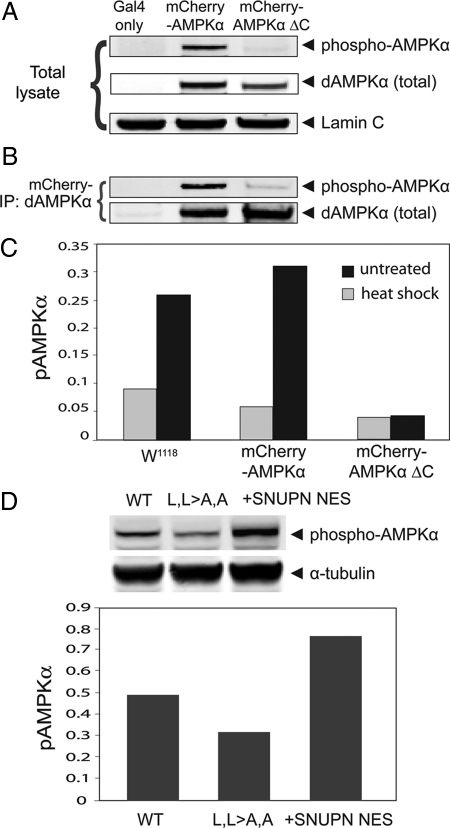
Figure 7.
Phosphorylation of the activation loop threonine (T184) AMPKα is reduced in a nuclear enriched form in Drosophila in vivo and increased in the cytoplasmic form in human cells in vitro. (A) Western blot of total lysates from transgenic Drosophila flies expressing Gal4 alone, mCherry-tagged full-length AMPKα, or mCherry-tagged truncated AMPKα (AMPKαΔC) probed for phospho-AMPKα, total AMPKα, and Lamin C (loading control). (B) Western blot of proteins immunoprecipitated with anti-AMPKα antibody from fly lysates from the above fly lines, probed for phospho- and total AMPKα. Both AMPKα protein constructs are overexpressed using the Gal4-UAS system, driven by Ubiquitin-Gal4. (C) Heat-shock stress (■) on live larvae induces increased activation loop threonine phosphorylation (pAMPKα) of wild-type (mCherry-AMPKα) and endogenous (W1118) AMPKα but not AMPKα missing the NES (AMPKαΔC). Standardized to Lamin C as a loading control. (D) Cytoplasmic AMPKα isoforms, wild-type (WT), and WT substituted with the snurportin (SNUPN) NES, demonstrate increased activation loop threonine phosphorylation compared with the nuclear (L,L>A,A) isoform. Standardized to tubulin as a loading control.