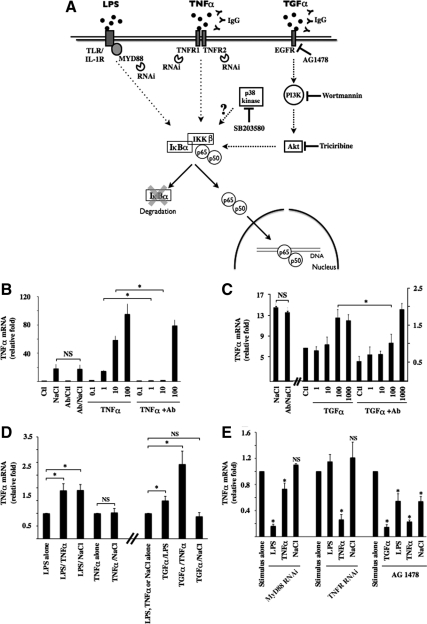
Figure 5.
NF-κB signaling by hypertonicity overlaps that elicited by TNF-α and TGF-α. (A) Schematic illustration of NF-κB activation by LPS, TNF-α, and TGF-α. Binding of each ligand to partner receptors initiates distinct transduction cascades that all ultimately lead to IKKβ activation. Under basal states, IκBα interacts with p65, retaining it in the cytoplasm. Once activated, IKKβ typically phosphorylates IκBα, leading to its degradation. This allows nuclear translocation of liberated p65-containing complexes, typically p65-p50 dimers. Tools used in this study to investigate the putative roles of target molecules in hypertonic NF-κB inducibility are shown. Also shown is p38 kinase whose role in NF-κB activation by hypertonicity was investigated. (B and C) Real-time PCR analysis of TNF-α transcript in cells challenged with hypertonic medium (NaCl, 500 mOsmol/kg), TNF-α (0.1–100 ng/ml), or TGF-α (1–1000 ng/ml) in the absence or presence of IgG against TNF-α (B) or TGF-α (C). Data are represented as fold induction over nonstimulated (Ctl) cells. (D) Real-time PCR analysis of TNF-α transcript in cells challenged with LPS, TNF-α, TGF-α (100 ng/ml), or hypertonic medium alone or simultaneously challenged with two stimuli. Data are represented as fold expression over cells challenged with LPS, TNF-α, or hypertonicity alone. (E) Real-time PCR analysis of TNF-α transcript in cells transfected with scrambled RNAi, RNAi against MyD88, or RNAi against TNFR1 and TNFR2 or cells treated with 10 μM of the EGFR antagonist AG1478. Cells were challenged with LPS, TNF-α, TGF-α, or hypertonic medium. Data are represented as fold expression over cells challenged with either stimulus alone.