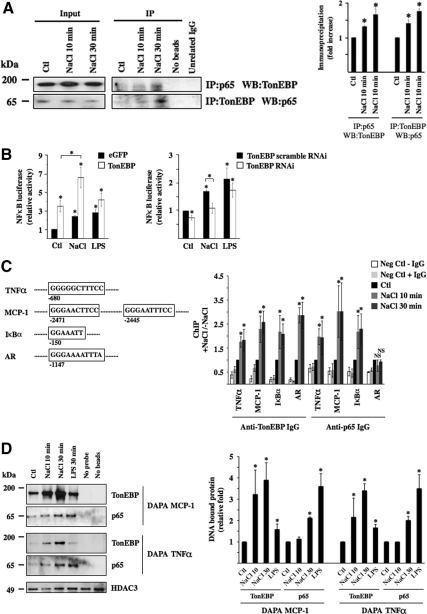
Figure 8.
TonEBP associates with p65 on the onset of hypertonic challenge. (A) Immunoblot against p65 and TonEBP immunoprecipitated by anti-TonEBP or anti-p65 IgG, respectively, or unrelated IgG (Na,K-ATPase α subunit) in cells challenged or not (Ctl) with hypertonic medium (NaCl) for 10 or 30 min. Also shown is the absence of bands from lysates precipitated in the absence of either agarose beads or IgG. Similar amounts of p65 and TonEBP between experimental conditions were loaded onto gels before immunoprecipitation (Input, corresponding to 5% of immunoprecipitated protein). Fold immunoprecipitated TonEBP and p65 over that of nonstimulated cells (Ctl) is shown at right. (B) NF-κB–driven luciferase activity in response to hypertonic (NaCl) or LPS challenge. Cells were transfected with a NF-κB-Luc plasmid described in Materials and Methods and cotransfected with cDNA encoding either eGFP or TonEBP (left) or with scrambled RNAi or RNAi against TonEBP (right). Data shown is represented as fold induction over nonstimulated cells transfected either with cDNA encoding eGFP or scrambled RNAi. (C) ChIP analysis. The localization of κB sites of mouse TNF-α, MCP-1, and IκBα promoters as well as the TonE site of mouse AR promoter chosen for analysis is shown. Localization is relative to the AUG start codon. Cells were challenged or not (Ctl) with hypertonic medium for 10 or 30 min before DNA fragmentation and immunoprecipitation using anti-TonEBP or p65 antibodies. Immunoprecipitated DNA was analyzed by real-time PCR using primers flanking κB sites of TNF-α, MCP-1, or IκBα promoters or the TonE site of the AR promoter. Data are represented as fold induction over nonstimulated cells. Negative controls consisted of DNA fragments precipitated in the absence of antibody or with anti- Na,K-ATPase α subunit IgG. (D) DAPA experiments were performed on nuclear extracts of cells challenged or not (Ctl) with hypertonic medium for 10 or 30 min or with LPS for 30 min. Precipitated protein by DAPA probes encompassing κB sites of MCP-1 or TNF-α promoters depicted in C was analyzed by immunoblot using anti-TonEBP or anti-p65 IgG. Equal loading was verified by immunoblot against histone deacetylase 3 (HDAC3). Negative controls consisted of protein precipitated in the absence of either a DAPA probe or beads. Precipitated TonEBP and p65 protein was quantified and is graphically represented as fold expression over control cells.