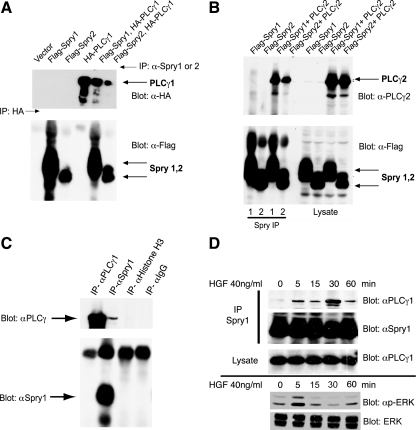
Figure 1.
PLCγ1 and PLCγ2 coimmunoprecipitate with Spry1 and Spry2. (A) HEK293 cells were transfected with plasmids expressing epitope-tagged Spry and PLCγ as indicated. Spry1 and Spry2 were immunoprecipitated with anti-Spry1 or anti-Spry2 antibody. HA-PLCγ1 was immunoprecipitated (lane 4) with anti-HA antibody. The immunoprecipitates were probed with anti-HA antibody to detect coimmunoprecipitated PLCγ1 (top) and with anti-FLAG antibody to detect Spry1 and Spry2 (bottom). (B) HEK293 cells were cotransfected with cDNAs encoding FLAG-tagged Spry1 or FLAG-tagged Spry2 and PLCγ2 as indicated. After 48 h, lysates were prepared, and immunoprecipitations were performed using specific anti-Spry1 or anti-Spry2 antibody (left). Total cell lysate (50 μg) was resolved in 10% SDS-polyacrylamide gel electrophoresis gel and probed with anti-PLCγ2 and anti-FLAG antibodies to show the expression level of the transfectants (right). (C) mIMCD3 cells growing in media containing 10% FBS were lysed and subjected to immunoprecipitation with anti-Spry1 and anti-PLCγ1 antibodies or IgG or anti histone H3 controls, followed by immunoblotting with anti-Spry1 or PLCγ1. (D) Murine IMCD3 cells were serum starved for 18 h followed by HGF (40 ng/ml) stimulation for the indicated time. Endogenous Spry1 and PLCγ1 interaction was demonstrated by immunoprecipitation with anti-Spry1 antibody and immunoblotting for PLCγ1. Total cell lysate was blotted with PLCγ1 to show the endogenous level of PLCγ1 in the cell lysates after stimulation with HGF. Bottom, lysate from a second experiment was immunoblotted for activated phosphorylated (p)-ERK and total ERK to show the time course of activation of signaling in response to HGF.