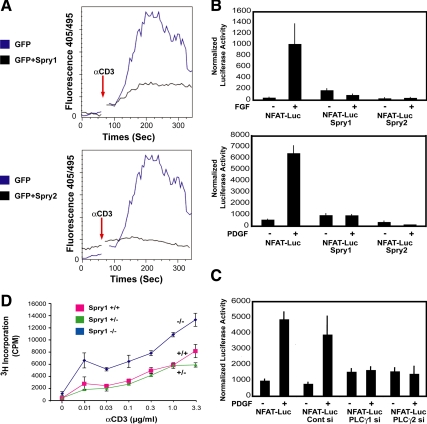
Figure 4.
Sprouty inhibits intracellular calcium mobilization and signaling in T-cells and fibroblasts. (A) Jurkat T-cells were cotransfected with a GFP expression vector (1 μg) along with either empty pcDNA (3 μg) or Spry1 or Spry2 expression vector (3 μg) as indicated. The cells were then loaded with indo-1 and after baseline measurements of 60 s and stimulated with anti-CD3 (3 mg/ml) antibody. Measurements were taken for up to 300 s. Intracellular calcium mobilization was analyzed by fluorescence-activated cell sorting analysis by monitoring the fluorescence emission ratio of the indo-1 bound to Ca2+ versus the free form at 405 and 495 nM, respectively. The experiment was performed three times with similar results obtained. (B) Spry1-inducible NIH 3T3 cells were transiently cotransfected with a reporter containing an NFAT-responsive element (5 μg) and Renilla (50 ng), starved for 24 h in media containing 0.2% FBS, and stimulated either with bFGF (20 ng/ml; top) or PDGF BB (20 ng/ml; bottom) for 4h. Spry1 was induced by doxycycline addition to the starvation media; Spry2 was expressed by transient transfection of a Spry2 expression plasmid in cells cultured in the absence of doxycycline. After growth factor addition, equal quantities of cell extracts (50 μg) were assayed using the Dual-Luciferase Assay kit (Promega), normalizing luciferase activity to Renilla luciferase activity. The experiments were repeated three times, and similar results were obtained. Luc, luciferase. (C) NIH 3T3 cells were treated with siGENOME SMARTpool directed against PLCγ1 or PLCγ2 for 24 h followed by transient transfection with NFAT luciferase reporter (5 μg) and Renilla (50 ng) plasmids by using FuGENE Transfection Reagent (GE Healthcare) in duplicate. Serum-starved (0.2% FBS-containing serum media) cells were either left unstimulated or stimulated with PDGF BB (20 ng/ml) for 4 h. Luciferase activity was assayed as described above. (D) T-cells from Spry1−/− mice were cultured in triplicate with the indicated amount of anti-CD3 antibody in combination with anti-CD28 (1 μg/ml) antibody for 72 h, and [3H]thymidine incorporation was quantified by liquid scintillation counting.