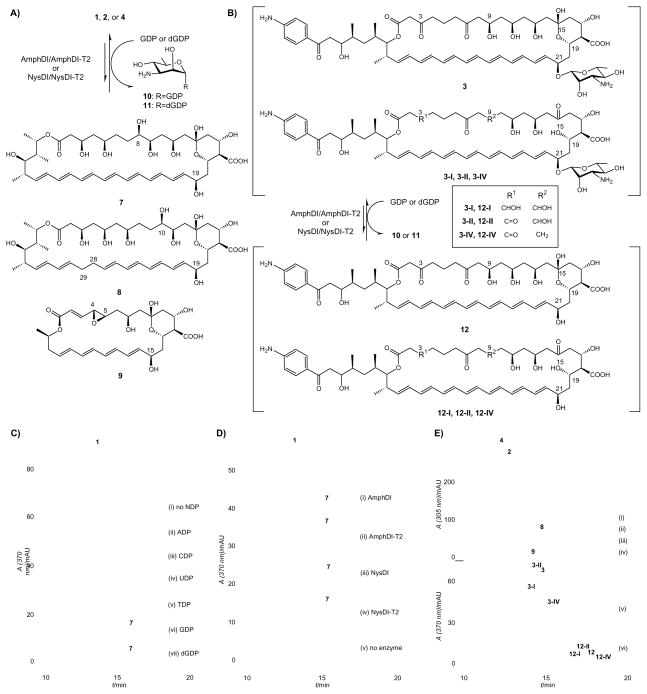
Figure 1. Polyene GT-catalyzed reverse reactions.
(A) Schematic of polyene GT-catalyzed conversion of 1, 2 or 4 to deglycosylated products 7, 8 or 9, respectively; (B) Schematic of polyene GT-catalyzed conversion of candicidin complex (3-I, 3-II, 3 and 3-IV) to deglycosylated complex (12-I, 12-II, 12 and 12-IV); (C) HPLC analyses of AmphDI-T2 NDP-specificity in GT-catalyzed reverse reactions. In this example, 20 μM AmB (1) was incubated with 5 μM of AmphDI-T2 without NDP (i) or in the presence of 1 mM of ADP (ii), CDP (iii), UDP (iv), TDP (v), GDP (vi) or dGDP (vii), at 30 °C overnight; (D) HPLC analyses of polyene GT-catalyzed reverse reactions with AmB (1) and different polyene GTs. For this study, 20 μM AmB (1) was incubated with 1 mM of GDP in the presence of 5 μM AmphDI (i), AmphDI-T2 (ii), NysDI (iii), NysDI-T2 (iv) or without GT (v), at 30 °C overnight; (E) HPLC analyses of AmphDI-T2 aglycon specificity in GT-catalyzed reverse reactions. In this study, 20 μM nystatin (2), 50 μM pimaricin (4) or 20 μM of candicidin complex (3-I, 3-II, 3 and 3-IV) were incubated with 1 mM GDP in the absence or presence of 5 μM AmphDI-T2: (i) 2, no enzyme (control), (ii) 2, AmphDI-T2, (iii) 4, no enzyme (control), (iv) 4, AmphDI-T2, (v) candicidin complex (3-I, 3-II, 3 and 3-IV), no enzyme (control), (iii) candicidin complex (3-I, 3-II, 3 and 3-IV), AmphDI-T2.