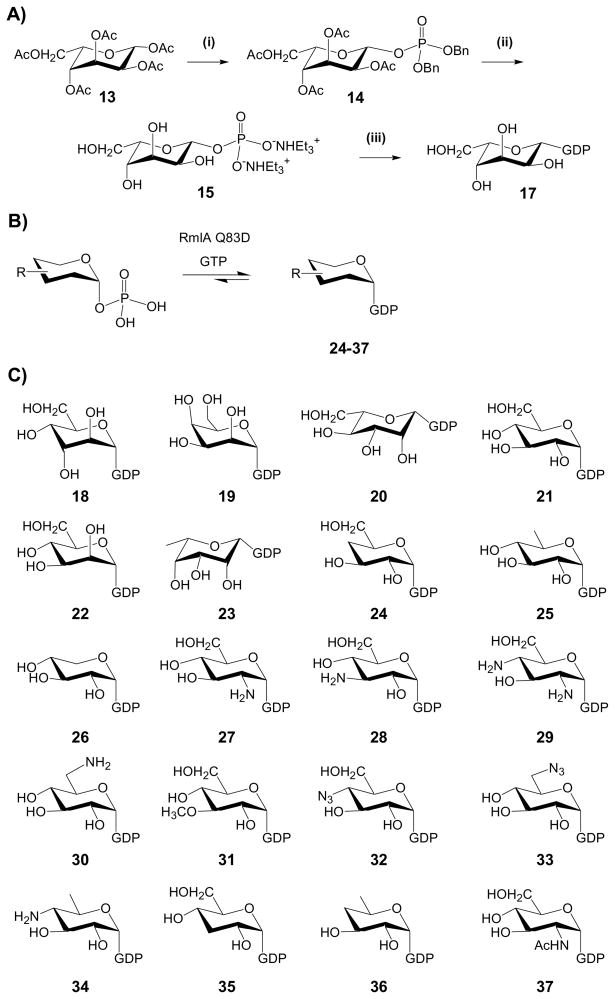
Scheme 2. Chemical and chemoenzymatic preparation of GDP-sugars.
(A) The chemical synthesis of GDP-L-β-gulose (17). (i) Ac2O/pyridine; HBr/AcOH; HPO2(OBn)2, CF3SO3Ag, Me3C5H2N/CH2Cl2; (ii) H2/Pd-C; AG 50W-X8 (Et3NH+); (iii) GDP-morpholidate (16) and 1H-tetrazole/pyridine. (B) The chemoenzymatic synthesis of GDP-sugars. Generally, 6 mM of chemically synthesized sugar-1 phosphate was incubated with 8 mM of GTP in the presence of 20 μM RmlA mutant Q83D. (C) GDP-sugars employed in this work. GDP-D-mycosamine (10) was generated via reverse GT-catalysis, GDP-D-glucose (18), GDP-D-mannose (19) and GDP-L-fucose (22) were commercially available; GDP-L-gulose (17), GDP-D-altrose (20), GDP-D-talose (21) and GDP-L-mannose (23) were chemically synthesized (scheme A) and GDP-sugars 24–35 were enzymatically synthesized (scheme B).