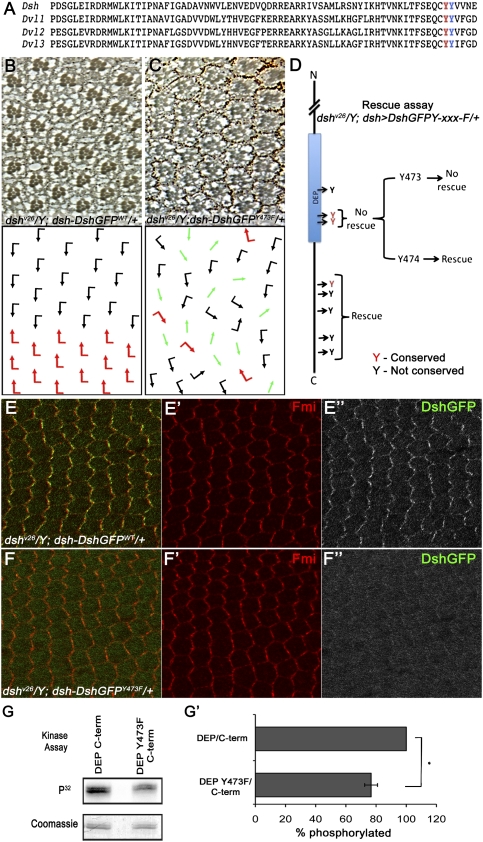
Figure 6.
Tyr473 in the DEP domain is essential for Dsh function in PCP signaling and is phosphorylated by Abl. (A) Protein sequence alignment of Dsh DEP domain from Drosophila Dsh and three mammalian Dvls, showing two conserved tyrosine residues (Tyr473 and Tyr474) within the DEP domain. (B–D) Rescue assay using the dsh-DshGFP expression cassette in a dsh-null background: (B) dshv26/Y; dsh-DshGFPWT/+ flies were fully rescued and their eyes displayed wild-type PCP features. Note that dsh-DshGFPWT fully rescued all aspects of the dshv26. (C) dshv26/Y; dsh-DshGFPY473F/+: Note that, while dsh-DshGFPY473F/+ rescued lethality (associated with canonical Wg signaling), it did not rescue the PCP function of Dsh, displaying classical PCP defects very similar to clones of dshv26 in the eye (as shown here) and the wing (not shown). (D) Schematic summary of dshv26/Y; dsh-DshGFPY-xxx-F rescues: Note that, except for a DshY473F (and DshY473.474F), all other Tyr mutations fully rescued lethality and PCP defects, behaving like Dsh-WT. Conserved Tyr residues are marked in red. (E–F) DshY473F affects PCP-specific membrane association of Dsh, as detected in 30 h APF (after puparium formation) pupal wing cells. (E–E″) dshv26/Y; dsh-DshGFP/+ stained with anti-Fmi (red in E,E′) and anti-GFP (DshGFP; green in E; grayscale in E″). Expression of dsh-DshGFP completely rescued the dshv26/Y mutant and displayed wild-type asymmetric membrane localization within the proximal–distal axis. (F–F″) dshv26/Y; dsh-DshGFPY473F/+ stained with anti-Fmi (red in F ,F′) and anti-GFP (DshGFP; green in F; grayscale in F″). (F,F″) Note the markedly reduced Dsh membrane association, which is critical for Dsh function in PCP signaling. (G,G′) In vitro kinase assay with purified Abl and DEP-Y473F/C-term (shown in G). The top panel shows an autoradiograph of a kinase assay, where DEP-Y473F/C-term shows reduced phosphorylation as compared with wild-type DEP/C-term. The bottom panel shows Coomassie staining of the same gel to reveal equal loading. (G′) Quantification of the of DEP/C-term and DEP-Y473F/C-term phosphorylation (ratio of DEP/C-term and DEP-Y473F/C-term phosphorylated to the total amount of DEP/C-term and DEP-Y473F/C-term loaded) by purified Abl kinase. Note the significant reduction in Abl phosphorylation with DEP-Y473F/C-term as compared with wild-type DEP/C-term. Values shown represent the average ± SD (n = 3). (*) P < 0.01, DEP-Y473F/C-term compared with the wild-type DEP/C-term.