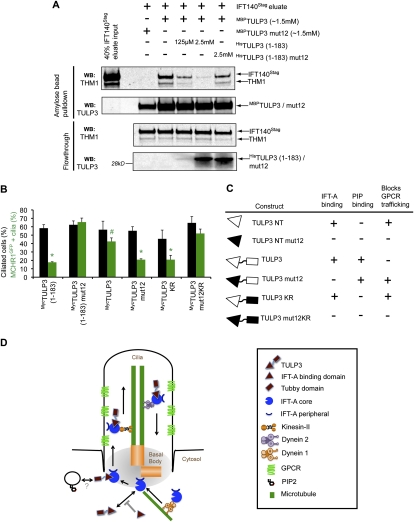
Figure 7.
Both IFT-A- and phosphoinositide-binding properties of TULP3 regulate GPCR-trafficking. (A) The TULP3 N terminus prevents recruitment of full-length TULP3 to an intact preformed IFT-A complex. PreScission eluates from TULP3 siRNA-treated IFT140LAP RPE cells were added to MBPTULP3 or MBPTULP3mut12 beads in the presence of HisTULP3 (1–183) or HisTULP3 (1–183) mut12. MBPTULP3 or MBPTULP3mut12-bound proteins and corresponding flowthroughs were immunoblotted for THM1 and TULP3. The anti-THM1 antibody cross-reacts with Stagfusion proteins, and allowed us to detect the IFT140Stag protein. For details, see the Supplemental Material. (B) RPE MCHR1GFP stable cells were transfected with the indicated myc-tagged TULP3 constructs and serum-starved for 30 h before fixing and immunostaining for myc, pericentrin, axonemal marker Glu-tubulin, and DNA. Percentages of total cilia and GFP-positive cilia in myc-positive cells were counted in at least three independent experiments. Error bars represent SEM. (*) P < 0.05 with respect to MCHR1GFP-positive cilia in MycTULP3 (1–183) mut12, MycTULP3, or MycTULP3mut12KR-expressing cells; (#) P < 0.05 with respect to MCHR1GFP-positive cilia in MycTULP3 (1–183) mut12-expressing cells. All other values are not significant with respect to each other. (C) Summary table of different TULP3 constructs and their effects on IFT-A binding, phosphoinositide binding, and GPCR trafficking. The mutant domains (for the image key, see D) are shown in black. (D) Model depicting the role of IFT-A and TULP3 in GPCR trafficking. IFT-A “core” complex associates with and provides ciliary access to TULP3. TULP3, in turn, is required for trafficking of certain ciliary-localized GPCRs. GPCR trafficking may be facilitated by loading of IFT-A onto preciliary vesicles (see also Sedmak and Wolfrum 2010) via the association of TULP3 with membrane phosphoinositides or novel interacting proteins. Dynein-1 also binds to the IFT-A complex, and may have roles in preciliary transport. Knocking down the IFT-A “core” complex proteins prevents TULP3 from localizing to the cilia, thereby inhibiting GPCR trafficking. Knocking down TULP3 affects ciliary localization of GPCRs, but not of IFT-A or IFT-B. Expression of the TULP3 N-terminal fragment prevents endogenous TULP3 from being recruited to the IFT-A complex, and possibly prevents loading of the IFT-A complex to the preciliary vesicles. See also Supplemental Figure S6.