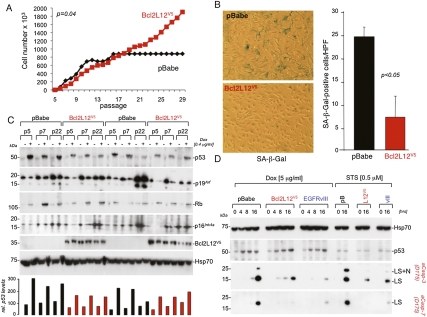
Figure 1.
Bcl2L12 inhibits passage-induced senescence and p53-dependent apoptosis. (A) MEF immortalization assays. pBabe and Bcl2L12-infected (wild-type) MEF cultures were serially passaged and cell numbers were determined. Shown is one representative growth curve documenting unabated growth of Bcl2L12-infected MEF cultures at >30 passages. (B) Staining for and quantification of senescence-associated β-galactosidase (SA-β-Gal) in representative pBabe and Bcl2L12 MEF lines. Histogram shows quantification of β-Gal-positive cells in pBabe versus Bcl2L12 transfectants; three high-power fields (HPFs) were counted. Data are represented as mean ± SD. (C) Molecular analysis of p53–Rb signaling components. pBabe and Bcl2L12 MEFs of indicated passages were treated with Dox (0.4 μg/mL) for 16 h to assess p53, p19Arf, and Rb protein levels. p53 proteins levels were quantified densitometrically. (D) Bcl2L12 potently blocked Dox-induced effector caspase activation. Cultures were treated with Dox (5 μg/mL) and STS (0.5 μM) for the indicated periods of time, and post-mitochondrial caspase-3 and caspase-7 activation was followed by Western blot analysis. Of note, p53 protein was induced in pBabe (pB), Bcl2L12 (L12V5), and EGFRvIII (vIII) astrocytic cultures upon Dox, but not STS, treatment. The migration positions of cleaved caspase-3 and caspase-7 species ([LS] large subunits; [LS+N] large subunits with N-peptide) are indicated. Hsp70 is shown as a loading control.