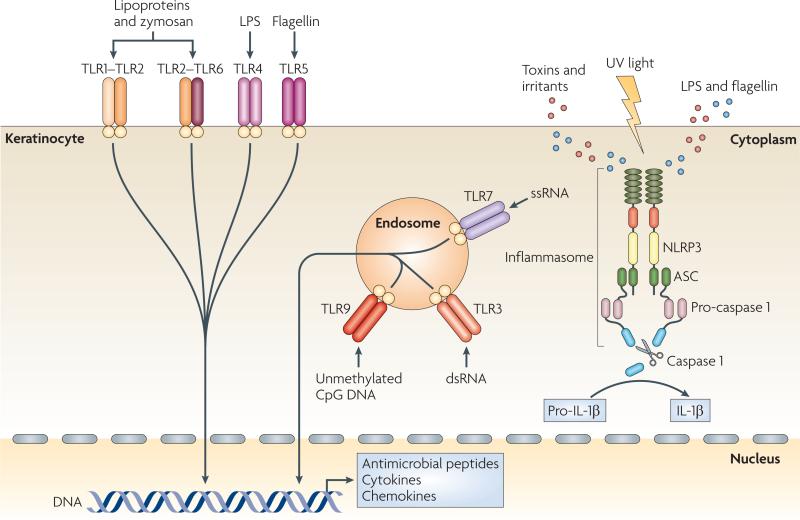
Figure 2. Keratinocytes as sensors of danger.
Keratinocytes are central skin sentinels and can recognize foreign and dangerous agents, for example pathogen-associated molecular patterns (PAMPs) of microbial origin and danger-associated molecular pattern (DAMPs), such as irritants and toxins, through Toll-like receptors (TLRs) and the inflammasome machinery. TLRs are transmembrane receptors that are present on the cell surface or on the surface of endosomal compartments. Lipopolysaccharide (LPS) stimulates TLR4; bacterial lipoproteins and fungal zymosan stimulate TLR1–TLR2 and TLR2–TLR6 heterodimers; bacterial flagellin activates TLR5; unmethylated CpG motifs present in DNA function as stimulators of endosomal TLR9; double-stranded RNA (dsRNA) activates endosomal TLR3; and single-stranded RNA (ssRNA) activates TLR7, the expression of which is induced by TLR3 triggering (not shown). PAMP recognition by TLRs leads to activation of host cell signalling pathways and subsequent innate and adaptive immune responses with antimicrobial peptide, cytokine and chemokine production. Keratinocytes also express NLR family, pyrin domain containing 3 (NLRP3), which belongs to the newly identified class of proteins encoded by the nucleotide-binding domain, leucine-rich repeat-containing (NLR) gene family. These proteins can recognize PAMPs that are in the cytoplasm (such as LPS and flagellin), DAMPs and ultraviolet (UV) light, and activate the inflammasome complex. This multimeric complex is formed by an NLR, an adaptor protein termed ASC (apoptosis-associated speck-like protein containing a caspase recruitment domain) and pro-caspase 1, and its assembly leads to the activation of caspase 1, which processes pro-interleukin-1β (pro-IL-1β) into biologically active IL-1β.