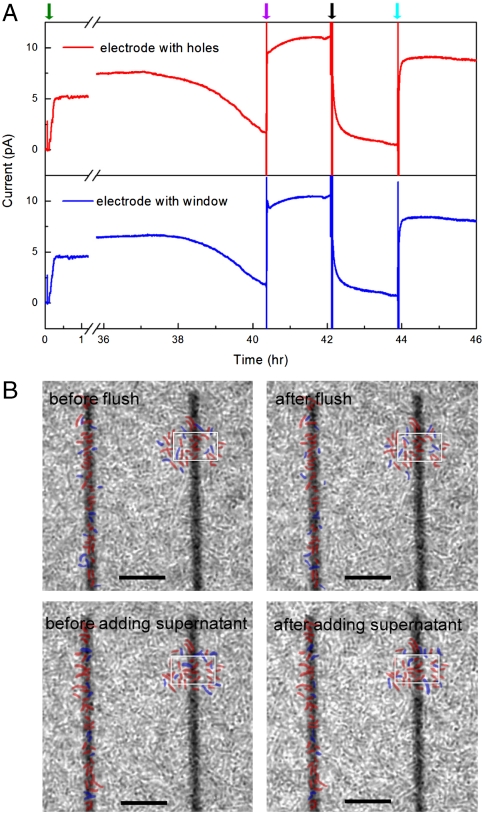
Fig. 4.
Current and cell imaging measurements at long times with biofilm formation. (A) Long-term short-circuit current measurement on electrodes with nanoholes (red) and large window (blue), whereas the green, purple, black and cyan arrows indicate cell addition, lactate addition, flush by fresh MM, and supernatant addition, respectively. (B) Phase-contrast images of cells/electrode before, after flush and supernatant addition. Positions of cells near the nanohole (Left) and window (Right) electrodes that did not and did shift position during solution exchanges are marked in red and blue, respectively. The window is marked in white for clarity in each image; scale bars are 10 μm. Specific details of solution exchanges to/from the measurement chamber are as follows: 15 μL of 2 M sodium lactate [diluted from 60% Sodium DL-lactate solution (Sigma-Aldrich)] was directly injected into measurement chamber (containing ∼1 mL solution), leading to final lactate concentration of ∼30 mM with minimal dilution of other species. For the flush with fresh MM, the supernatant in measurement chamber was removed with a syringe, and then 1 mL nitrogen purged fresh MM (containing 30 mM lactate) was added to the chamber, where the addition of nitrogen purged fresh MM was repeated twice to ensure removal of mediators in the measurement chamber. The original supernatant, which was centrifuged at 3,000 rpm for 5 min to remove planktonic cells, was returned to the measurement chamber in the final exchange after removing the previous fresh MM by syringe. The original supernatant was diluted during solution exchange due to the incomplete removal of fresh media.