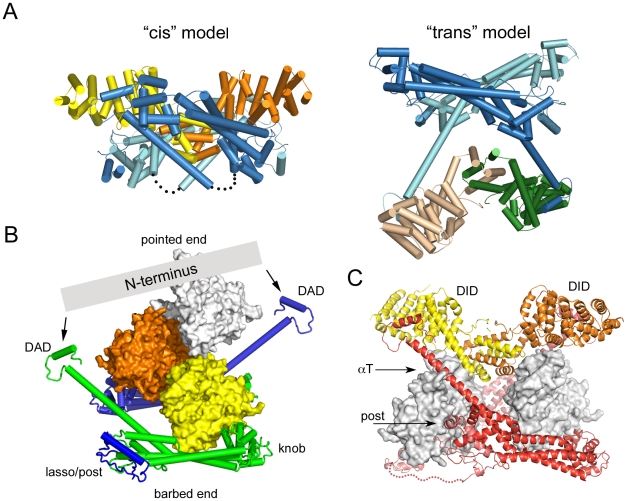
Figure 6. Speculations on mechanisms of autoinhibition in intact diaphanous-related formins.
A, The autoinhibited tetramer structure suggests two general models for an autoinhibited dimer. Structures are colored as in Figure 1B. In the cis model (left), the N-terminus packs into the FH2 domain, as in the top half of the tetramer. This arrangement would require the αT Helix of each hemidimer to fold back (as indicated by the dotted lines) in order to allow binding of the DAD segment to the adjacent N-terminus. In this model, formation of the tetramer represents a “domain swap” in which the DAD and C-terminal portions of αT exchanged positions with the corresponding portions of the juxtaposed C-terminus to create the interlocked tetramer. The trans model maintains the DID/DAD interaction found in the tetramer; it is simply the complex of the C-terminal fragment with the N-terminus that engages its DAD region (e.g., the upper FH2 domain with the lower N-terminus). B, The structure of the FH2+DAD region of mDia1 is docked on actin as in the structure of the Bni1/actin complex. Simultaneous engagement of the DAD segments by the dimeric N-terminus in almost any conceivable conformation or orientation (represented by the gray bar) would be expected to block addition of more than one or two actin subunits, and therefore to lead to inhibition of productive nucleation and processive capping. C, Actin subunits are docked on the knob subdomain of each bridge element of an FH2 dimer, with the N-terminus engaged as in the trans model. Actin subunits were docked on mDia1 by superposition of its knob subdomain with that of Bni1 in the Bni1/actin structure [22]. The superposition and docking reveals minor steric clashes between actin and the αT and post regions of the adjacent bridge element, but these could likely be relieved by flexibility in the FH2 linkers (shown as dotted lines), the loops that lead into the DAD domains, and in the N-terminus itself. However, note the large gap between actin subunits. Distortion of the FH2 dimer by engagement of the N-terminus is likely to interfere with stabilization of filament-like interactions between actin subunits, which are thought to be required for effective nucleation.